Slow release medical composition for treating anxiety disorders and preparation method of slow release medical composition
A technology for sustained-release medicine and composition, applied in the field of medicine, can solve the problems of hindering the development of tandospirone citrate sustained-release pharmaceutical composition, high price of hydroxypropyl methylcellulose, poor patient taking compliance, and the like, Achieve the effects of low preparation cost, low equipment dependence, and easy operation of the production method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
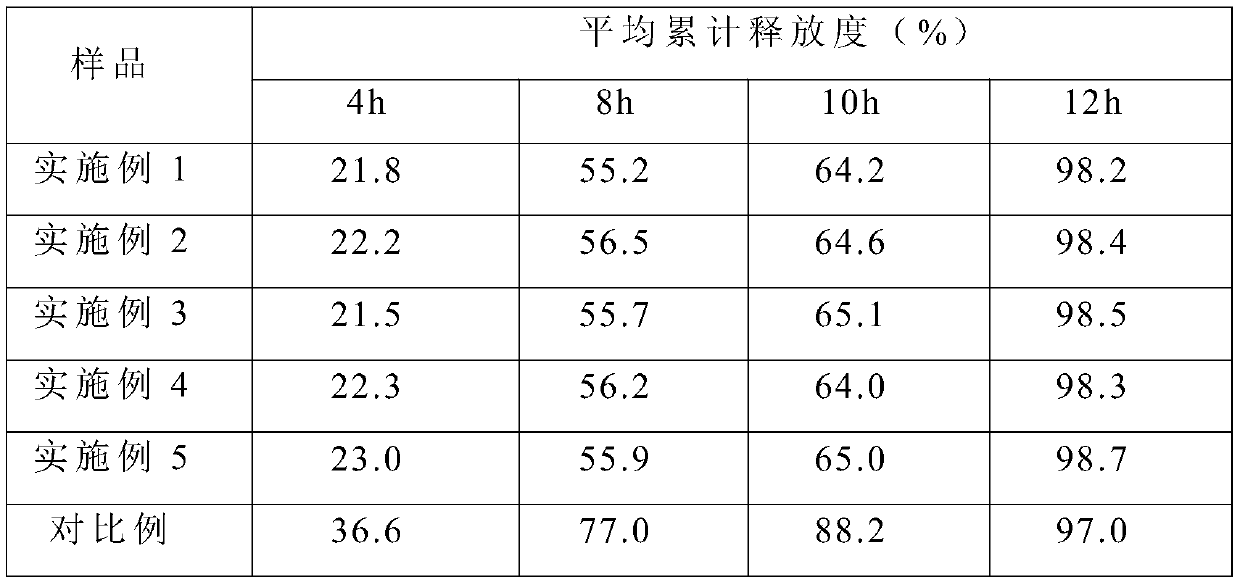
Examples
Embodiment 1
[0028] A slow-release pharmaceutical composition for treating anxiety, made of the following raw materials in parts by weight: 45 parts of tandospirone citrate, 10 parts of 4-aminobutyric acid alginic acid co-modified copolymer, 5 parts of lactose, 3 parts of povidone, 1 part of magnesium stearate, 3 parts of Chinese herbal medicine extract, 5 parts of water; the copolymer is made by allyl β-D-glucopyranoside, sweet glucoside, 1-vinyl- 3-Butylimidazolium chloride is prepared by free radical copolymerization.
[0029] The preparation method of described 4-aminobutyric acid alginic acid co-modified copolymer comprises the steps:
[0030] Preparation of step S1 copolymer: 10 g of allyl β-D-glucopyranoside, 10 g of sweet glucoside, 2 g of 1-vinyl-3-butyl imidazolium chloride salt, and 0.1 g of azobisisobutyronitrile were added to In 60g of dimethyl sulfoxide, stirred and reacted at 70°C under nitrogen atmosphere for 6 hours, then precipitated in hexane, removed the solvent by rot...
Embodiment 2
[0035] A slow-release pharmaceutical composition for treating anxiety, made of the following raw materials in parts by weight: 47 parts of tandospirone citrate, 12 parts of 4-aminobutyric acid alginic acid co-modified copolymer, 7 parts of sucrose, 4 parts of starch, 2 parts of talcum powder, 4 parts of Chinese herbal medicine extract, and 7 parts of water; Imidazolium chloride is prepared by free radical copolymerization.
[0036] The preparation method of described 4-aminobutyric acid alginic acid co-modified copolymer comprises the steps:
[0037] Preparation of step S1 copolymer: 10 g of allyl β-D-glucopyranoside, 10 g of sweet glucoside, 2 g of 1-vinyl-3-butyl imidazolium chloride salt, and 0.15 g of azobisisoheptanonitrile were added to In 75g of N,N-dimethylformamide, stir and react at 73°C under helium gas atmosphere for 6.5 hours, then precipitate in hexane, remove the solvent by rotary evaporation, and place in a vacuum oven at 73°C Dry to constant weight;
[0038...
Embodiment 3
[0042] A slow-release pharmaceutical composition for treating anxiety, which is made of the following raw materials in parts by weight: 50 parts of tandospirone citrate, 13 parts of 4-aminobutyric acid alginic acid co-modified copolymer, 8 parts of manna, 4 parts of microcrystalline cellulose, 3 parts of silicon dioxide, 4 parts of Chinese herbal medicine extract, and 8 parts of water; 3-Butylimidazolium chloride is prepared by free radical copolymerization.
[0043] The preparation method of described 4-aminobutyric acid alginic acid co-modified copolymer comprises the steps:
[0044] Preparation of step S1 copolymer: Add 10g of allyl β-D-glucopyranoside, 10g of sweet glucoside, 2g of 1-vinyl-3-butylimidazolium chloride salt, and 0.2g of azobisisobutyronitrile into In 80g of N-methylpyrrolidone, stir and react at 76°C for 7 hours under a neon gas atmosphere, then precipitate in hexane, remove the solvent by rotary evaporation, and dry in a vacuum oven at 75°C to constant wei...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com