Copolymerization micelle drug-loading nanoparticle and application thereof
A drug-loaded nanometer and micelle technology, which can be used in antitumor drugs, drug combinations, pharmaceutical formulations, etc., can solve the problems of complex preparation process, high cost, and poor tumor cell killing effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A copolymerized micelle drug-loaded nanoparticle disclosed in Example 1, which comprises an uncharged hydrophilic polymer chain block, an uncharged hydrophobic polymer chain block, and a cationic polymer chain block A block copolymer, the non-charged hydrophilic polymer chain block and the cationic polymer chain block both ends of the non-charged hydrophobic polymer chain block through chemical bonds, the cationic polymer The chain blocks are grafted with lipid ligands, wherein the uncharged hydrophilic polymer chain block is polyethylene glycol, and the uncharged hydrophobic polymer chain is ε-caprolactone, and the cationic The polymer chain block is polylysine, and the lipid ligand is cholesterol, forming a three-block copolymer poly(ethylene glycol)-poly(ε-caprolactone)-poly(L-lysine) from The assembled micellar system nano drug-loaded particle is recorded as mPEG-bPCL-b-PLL / Chol, and its chemical equation is as follows:
[0029]
[0030] As an improved specific ...
Embodiment 2
[0032] A method for preparing copolymerized micelles drug-loaded nanoparticles disclosed in Example 2,
[0033] Preparation of mPEG-b-PCL-b-PLL polymer: Dissolve appropriate amount of mPEG and ε-CL monomer in toluene, blow nitrogen gas, add catalyst stannous zincate, stir and react at 120°C for 24 hours under nitrogen protection, and obtain mPEG-b-PCL-OH; Take 3g dry mPEG-b-PCL-OH powder, dissolve it in 15ml chloroform, add TEA and MsCl, stir and react at 0℃ for 12 hours, extract and lyophilize, redissolve in DMF solvent, add Diazonium salt, reaction at room temperature for 48 hours to obtain mPEG-b-PCL-N 3;mPEG-b-PCL-N 3 with propargyl-PZLL, CuCl 2 (catalyst), NaVc was dissolved in DMF, under nitrogen protection, microwave reaction was used for 30 minutes to obtain mPEG-b-PCL-b-PZLL; the obtained product was added with TFA and hydrogen bromide, and reacted at 0°C for 40 minutes to obtain the final mPEG- b-PCL-b-PLL polymer. The obtained final product was extracted with et...
Embodiment 3
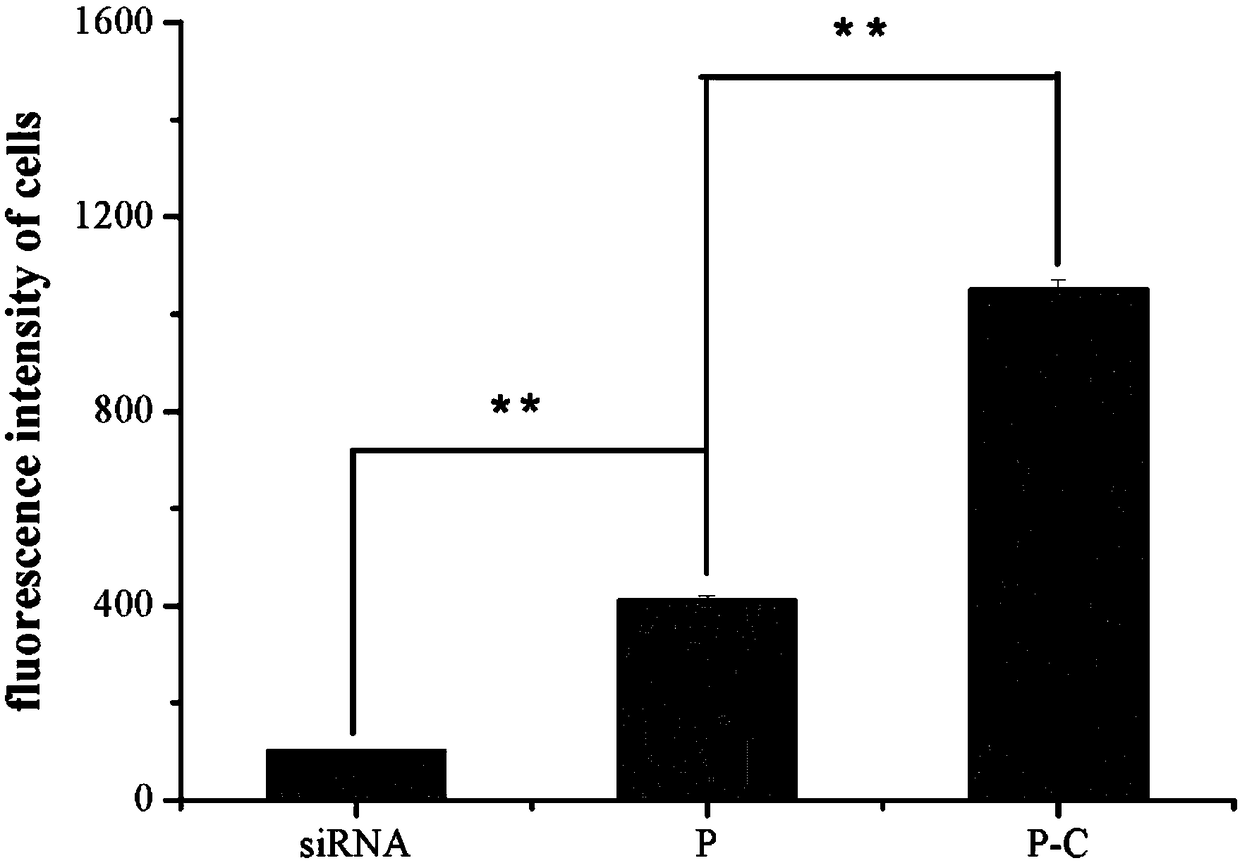
[0036] Example 3 discloses the loading method of doxorubicin and siRNA loading the above-mentioned copolymerized micellar drug-loaded nanoparticles. Dissolve mPEG-bPCL-b-PLL / Chol and doxorubicin in 1ml of tetrahydrofuran, and stir at 20°C for 1 Hours, self-assembled to form nanomicelles, recorded as PD; add 1ml double distilled water dropwise, continue to stir for 1 hour, extract micelles, remove THF; add 1ml siRNA solution to form co-loaded doxorubicin and siRNA micelles drug-loaded nanoparticles , recorded as PD / siRNA.
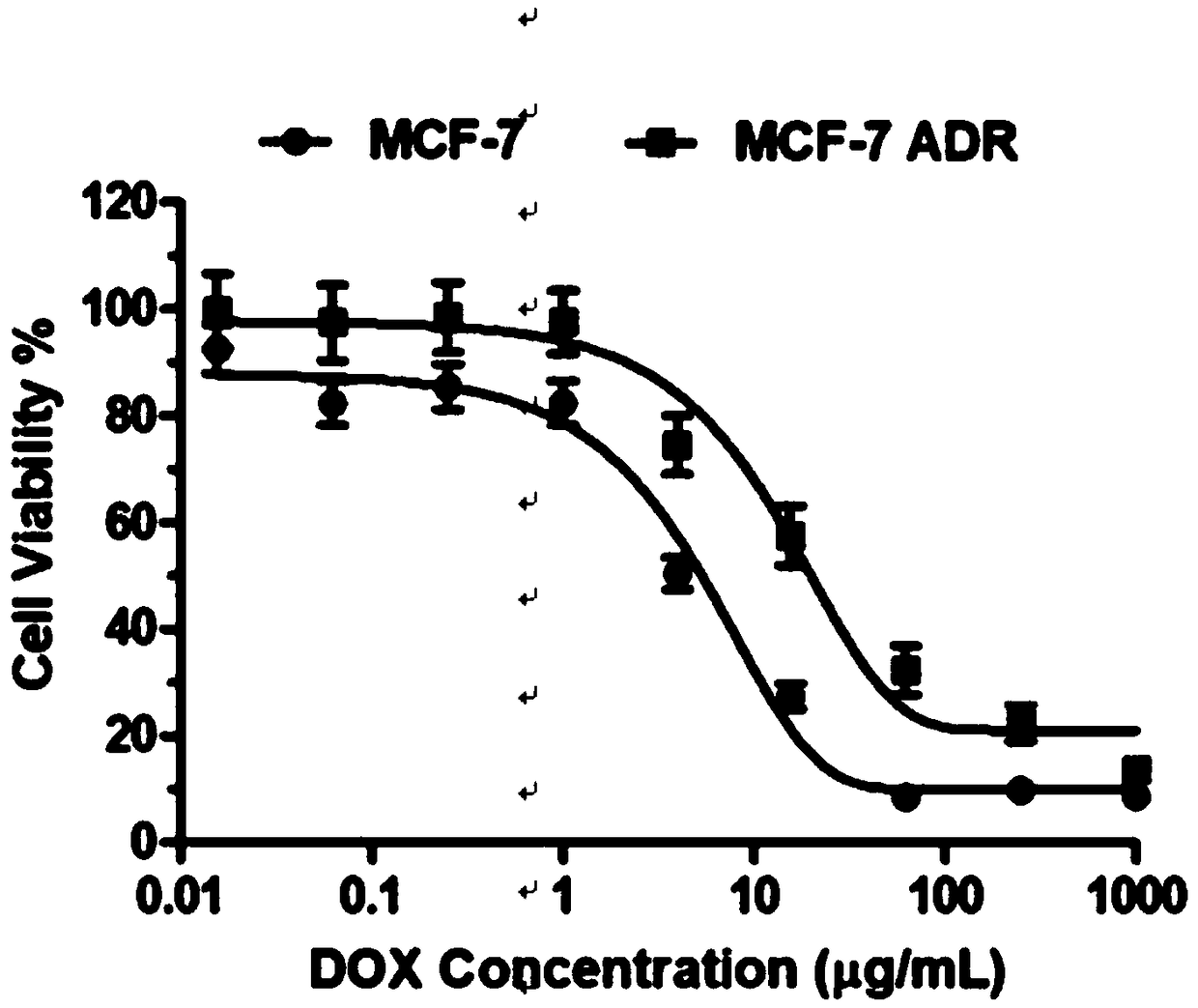
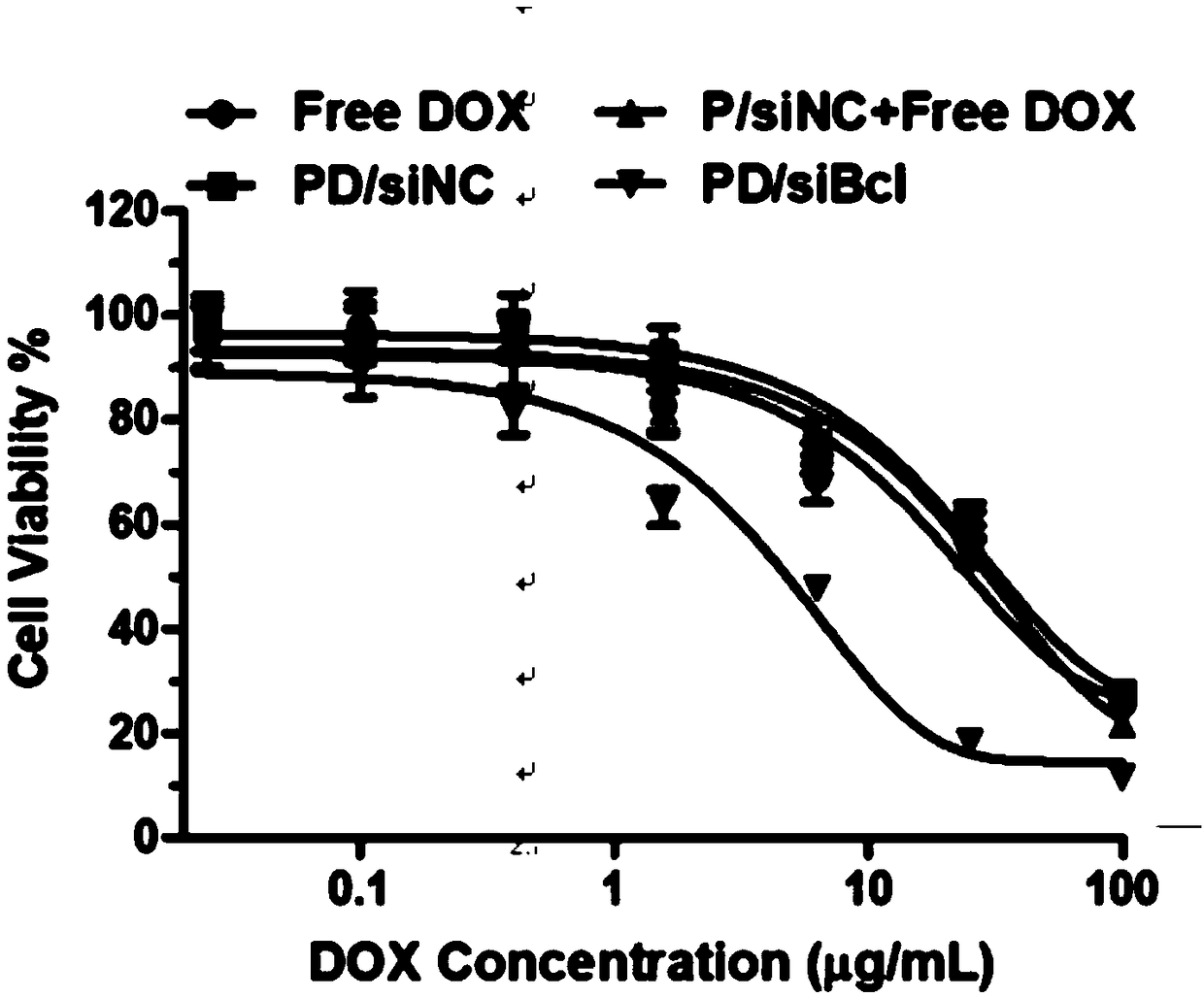
[0037] The micellar nano drug-loaded particles prepared according to the above method can selectively release siRNA and doxorubicin in an acidic environment. The results of the release experiment showed that under neutral conditions (pH=7.4), the cumulative release of doxorubicin / siRNA and doxorubicin was not much different, and the cumulative release amount was only 50%. While under acidic conditions (pH=5.5), the cumulative release of doxorubicin is 80%. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com