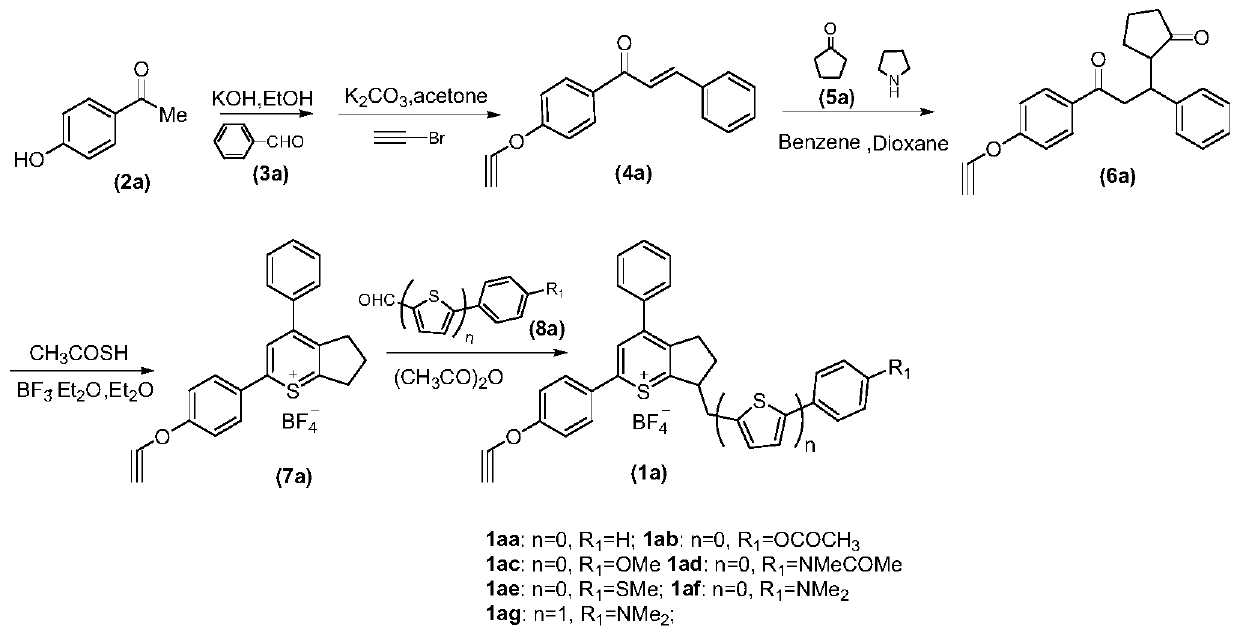
A near-infrared second region fluorescent compound capable of targeting mitochondria, its preparation method and application
A fluorescent compound and compound technology, applied in chemical instruments and methods, organic chemistry, luminescent materials, etc., to achieve the effects of high industrial production value, simple synthesis route and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: the preparation of compound 4a
[0057] Compound 2a (1.36 g, 10 mmol) was dissolved in 20 mL of ethanol, 20% KOH (20 mL) solution was added and reacted at room temperature for 10 minutes. Benzaldehyde 3a (1.16 g, 11 mmol) was then added to the mixture, and the reaction was stirred at room temperature overnight. After cooling to room temperature, the reaction solution was adjusted to pH=3 with 2M HCl solution. The yellow intermediate chalcone formed was collected by filtration. Add chalcone (1.12g, 5mmol), potassium carbonate (1.52g, 11mol) and acetone (10mL) into the reaction vessel, stir at room temperature for 10 minutes, then add 3-bromopropyne (0.654g, 5.5 mol) was heated to reflux and stirred for 4 hours. The mixture was then filtered and the filtrate was concentrated under reduced pressure to afford product 4a (1.27 g, 97% yield).
[0058] The structure determination data of compound 4a are as follows:
[0059] 1H NMR (400MHz, Acetone-d6) δ8.24–...
Embodiment 2
[0061] Embodiment 2: the preparation of compound 6a
[0062] Cyclopentanone (1.2 g, 14.3 mmol) and tetrahydropyrrole (1.02 g, 14.3 mmol) were dissolved in benzene (20 mL), and the solution was heated to 100° C. and stirred for 4 hours. After cooling to room temperature, the benzene was removed under reduced pressure. Then compound 4a (2.5 g, 9.53 mmol) and anhydrous 1,4-dioxane (20 mL) were added to the above reaction solution, and the solution was heated under reflux for 6 hours. After the reaction was complete, water (40 mL) was added to the mixture, and extracted with ethyl acetate (50 mL×3). After that, the organic layer was washed with saturated brine. The combined organic layers were dried over anhydrous magnesium sulfate, filtered and concentrated. The solvent was then removed in vacuo and the crude product was purified by silica gel column chromatography (2.17 g, yield 66%).
[0063] The structure determination data of compound 6a are as follows:
[0064] 1H NMR (...
Embodiment 3
[0066] Embodiment 3: the preparation of compound 7a
[0067] Compound 6a (1.2g, 3.46mmol) was dissolved in diethyl ether (10mL) and stirred for 10 minutes, thioacetic acid (659mg, 8.66mmol) and boron trifluoride ether (2.46g, 17.32mmol) were added to the mixture in Heat at reflux for 3 hours. After cooling to room temperature, the reaction mixture was quenched with water, and excess diethyl ether was added to the solution. The mixture was then stirred at room temperature to precipitate a pale yellow solid (400 mg, yield 50%).
[0068] The structure determination data of compound 7a are as follows:
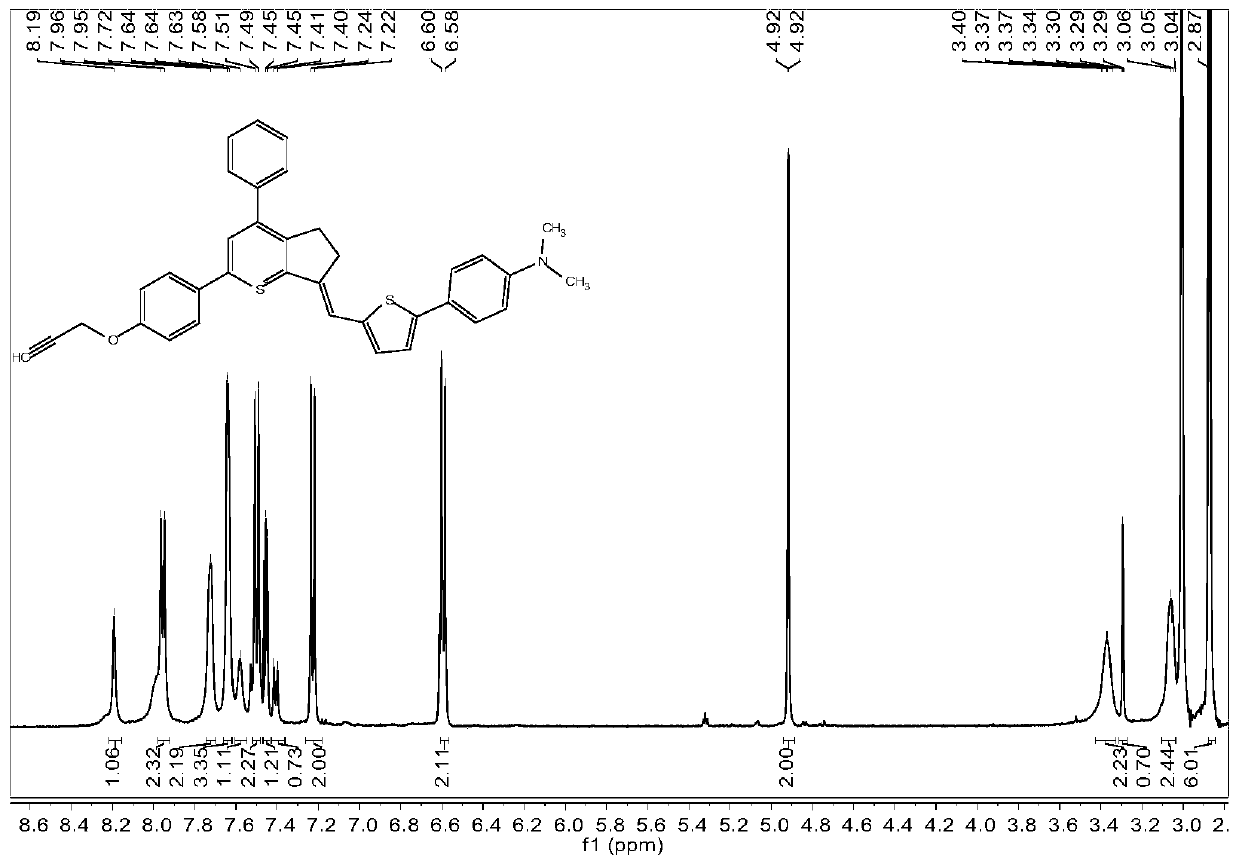
[0069] 1H NMR (400MHz, Acetonitrile-d3) δ8.63(s, 1H), 8.01(d, J=9.1Hz, 2H), 7.85–7.76(m, 2H), 7.76–7.63(m, 3H), 7.29( d, J=8.9Hz, 2H), 4.92(d, J=2.4Hz, 2H), 3.67(t, J=7.6Hz, 2H), 3.34(t, J=7.4Hz, 3H), 2.95(t, J=2.5Hz,1H),2.37(p,J=7.5Hz,2H).
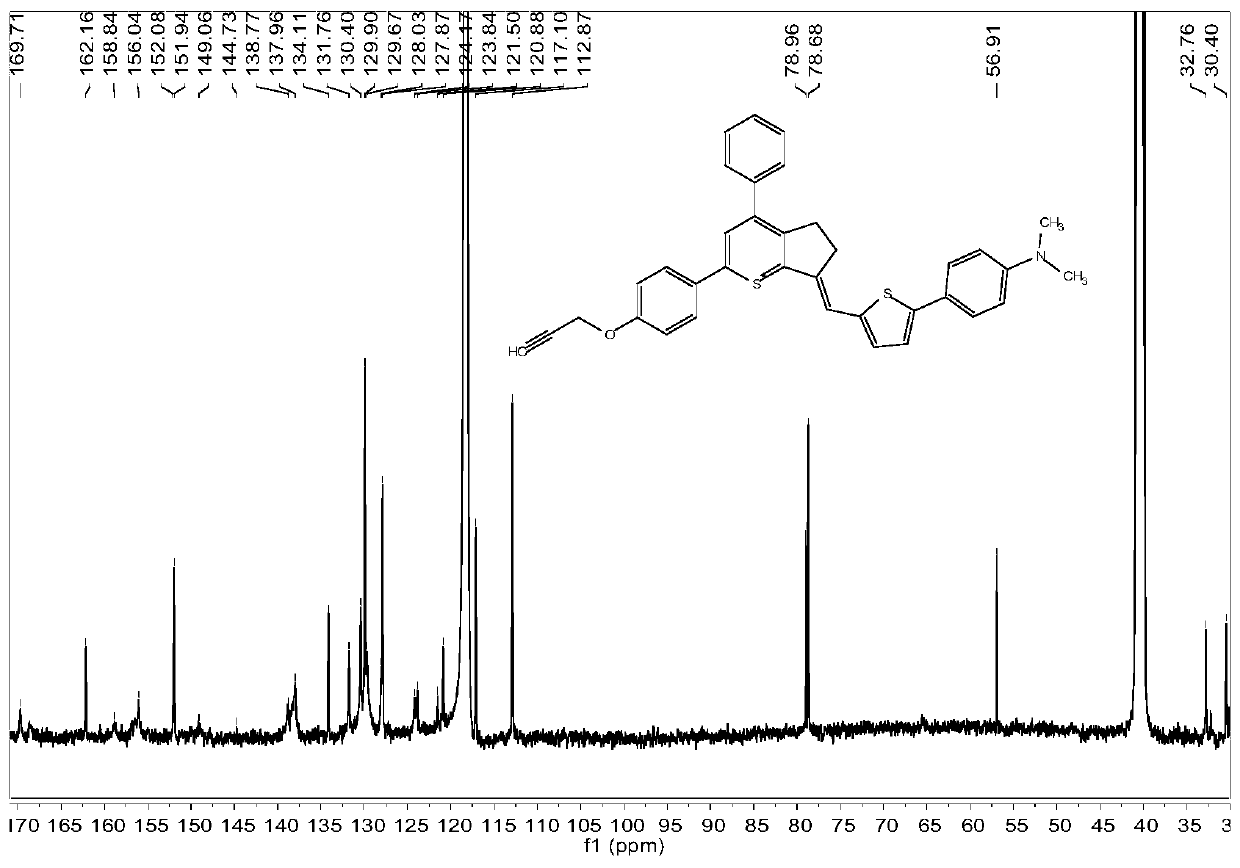
[0070] 13C NMR (101MHz, Acetonitrile-d3) δ174.5, 165.7, 161.9, 159.4, 149.5, 137.2, 132.5, 131.7, 130.3, 129.3, 129.2, 127.3, 116.6, 77....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com