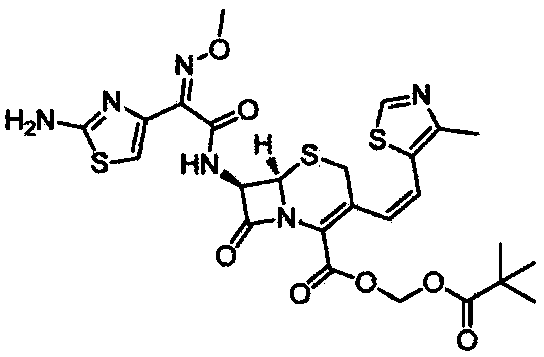
Preparation method of cefditoren pivoxil
A technology of cefditoren pivoxil and phosphate ester, which is applied in the field of medicine, can solve the problems of messy reaction, difficult purification process, and many by-products, and achieve the effects of mild reaction conditions, high product purity, and high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
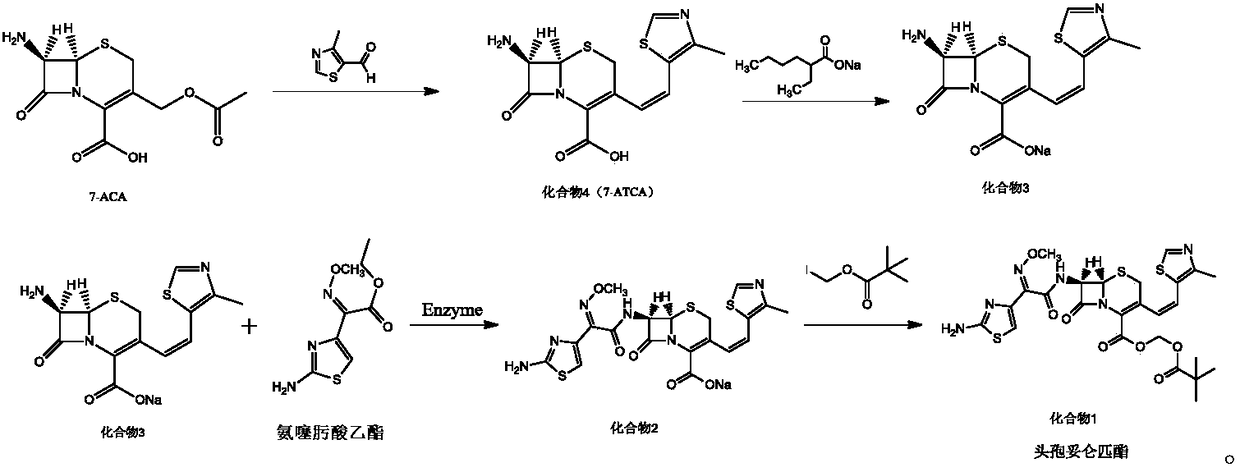
[0026] Embodiment 1: the preparation of compound 4
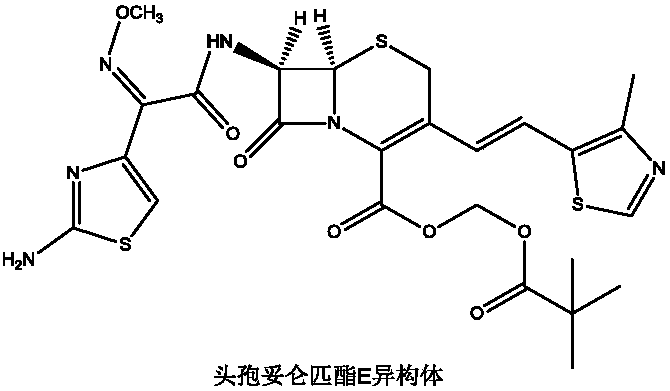
[0027] Under nitrogen protection, 100mL of benzene, 40mmol of 7-ACA, 48mmol of hexamethyldisilazane, and 0.5mmol of pyridine were successively added to the reaction flask, stirred and heated to reflux for 2 hours, cooled to 0°C, and three Methyl iodosilane (TMSI) 44mmol, keep warm for 1h, add 38mmol of trifluoroethoxy phosphate, continue to react for 1h, add 36mmol of sodium hexamethyldisilazide, 60mmol of 15-crown-5, heat and reflux for 30min, cool to At room temperature, 38 mmol of 4-methylthiazole-5-carbaldehyde was added and reacted at room temperature for 6 h. After the reaction was completed, 100 mL of methanol was added to precipitate a solid, which was filtered, washed with 50 mL of THF, and dried under reduced pressure at 35°C to obtain 10.52 g of compound 4 with a yield of 85.48% and a purity of 99.88%, without E isomer.
Embodiment 2
[0028] Embodiment 2: the preparation of compound 4
[0029] Under the protection of nitrogen, add 100mL of benzene, 40mmol of 7-ACA, 40mmol of hexamethyldisilazane and 0.5mmol of pyridine in turn to the reaction flask, stir and heat up to reflux for 2h, cool the reaction solution to 0°C, drop three Methyl iodosilane (TMSI) 40mmol, keep warm for 1h, add 32mmol of trifluoroethoxy phosphate, continue the reaction for 1h, add 32mmol of sodium hexamethyldisilazide, 40mmol of 15-crown-5, heat and reflux for 30min, cool to At room temperature, 32 mmol of 4-methylthiazole-5-carbaldehyde was added and reacted at room temperature for 6 h. After the reaction was complete, 100 mL of methanol was added to precipitate a solid, which was filtered, washed with 50 mL of THF, and dried under reduced pressure at 35°C to obtain 8.53 g of compound 4 with a yield of 82.04%, a purity of 99.57%, and E-isomer 0.14%.
Embodiment 3
[0030] Embodiment 3: the preparation of compound 4
[0031]Under the protection of nitrogen, add 100mL of benzene, 40mmol of 7-ACA, 56mmol of hexamethyldisilazane, and 0.5mmol of pyridine in sequence in the reaction flask, stir and raise the temperature to reflux for 2h, cool the reaction solution to 0°C, dropwise add three Methyl iodosilane (TMSI) 48mmol, keep warm for 1h, add 44mmol of trifluoroethoxy phosphate, continue the reaction for 1h, add 40mmol of sodium hexamethyldisilazide, 80mmol of 15-crown-5, heat and reflux for 30min, cool to At room temperature, 44 mmol of 4-methylthiazole-5-carbaldehyde was added and reacted at room temperature for 6 h. After the reaction was complete, 100 mL of methanol was added to precipitate a solid, which was filtered, washed with 50 mL of THF, and dried under reduced pressure at 35°C to obtain 10.75 g of compound 4 with a yield of 82.83%, a purity of 99.64%, and E-isomer 0.09%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com