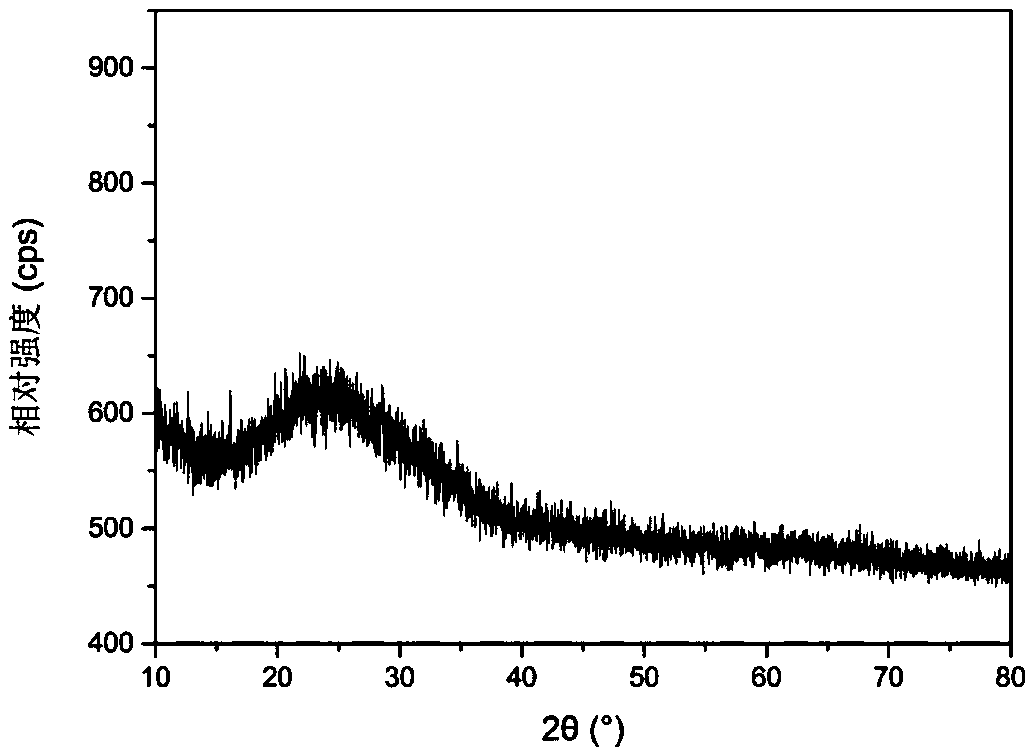
Preparation method of amorphous boron powder
A technology of amorphous boron powder and silicon powder, applied in the direction of boron, boron/boride, etc., can solve the problems of high requirements on anticorrosion of environmental protection facilities and equipment, inability to realize industrialized production, low added value of by-products, etc., to improve thermodynamic conditions , the effect of broadening the source of raw materials and narrowing the particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A preparation method of amorphous boron powder. The preparation method described in this embodiment is:
[0028] According to the mass ratio of reducing agent: boron-containing compound: molten salt of alkali metal compound: 1.0: (1.5-2.5): (3.5-8.0), the reducing agent, the boron-containing compound and the alkali metal-containing compound are melted The salt is mixed evenly, heat-treated in a protective atmosphere at 1000-1300°C for 6-8 hours, then dissolved in water at 95-200°C, filtered, washed and dried to obtain amorphous boron powder.
[0029] The reducing agent is silicon powder, or aluminum powder, or a mixture of silicon powder and aluminum powder.
[0030] The boron-containing compound is at least one of boron oxide, boric acid, sodium tetraborate, potassium tetraborate and lithium tetraborate.
[0031] The molten salt of the alkali metal compound is more than one of sodium hydroxide, sodium carbonate, sodium silicate, potassium hydroxide, potassium carbona...
Embodiment 2
[0033] A preparation method of amorphous boron powder. The preparation method described in this embodiment is:
[0034] According to the mass ratio of reducing agent: boron-containing compound: molten salt of alkali metal compound: 1.0: (1.5-2.5): (3.5-8.0), the reducing agent, the boron-containing compound and the alkali metal-containing compound are melted The salt is mixed evenly, heat-treated in a protective atmosphere at 1000-1300°C for 6-8 hours, then dissolved with an alkali solution at 95-200°C, filtered, washed and dried to obtain amorphous boron powder.
[0035] The reducing agent is silicon powder.
[0036] The boron-containing compound is one of boron oxide, boric acid, sodium tetraborate, potassium tetraborate and lithium tetraborate.
[0037] The molten salt containing alkali metal compound is one of sodium hydroxide, sodium carbonate, sodium silicate, potassium hydroxide, potassium carbonate, potassium silicate, lithium hydroxide, lithium carbonate, lithium si...
Embodiment 3
[0040] A preparation method of amorphous boron powder. The preparation method described in this embodiment is:
[0041] According to the mass ratio of reducing agent: boron-containing compound: molten salt of alkali metal compound: 1.0: (1.5-2.5): (3.5-8.0), the reducing agent, the boron-containing compound and the alkali metal-containing compound are melted The salt is mixed evenly, heat treated in a protective atmosphere at 1000-1300°C for 6-8 hours, then dissolved with an alkali solution at 95-200°C, filtered, washed and dried to obtain amorphous boron powder.
[0042] The reducing agent is aluminum powder.
[0043] The boron-containing compound is a mixture of boron oxide, boric acid, sodium tetraborate, potassium tetraborate and lithium tetraborate.
[0044] The molten salt of the alkali metal compound is sodium hydroxide, sodium carbonate, sodium silicate, potassium hydroxide, potassium carbonate, potassium silicate, lithium hydroxide, lithium carbonate, lithium silica...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com