Fusion protein of porcine pseudorabies virus and preparation method, application and vaccine of fusion protein
A porcine pseudorabies virus, fusion protein technology, applied in antiviral immunoglobulins, veterinary vaccines, biochemical equipment and methods, etc., can solve the problems of limited immune efficacy, long production cycle, high cost, and achieve good immunogenicity , low preparation cost, good immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] A method for preparing the above fusion protein, comprising expressing the gene encoding the fusion protein in a mammalian expression system. The preparation method can realize the high-quality expression of the above-mentioned fusion protein in large quantities, and is simple to operate and suitable for large-scale production. The mammalian expression system can be, for example but not limited to, HEK 293-F cells, HEK 293-E cells, HEK 293-T cells, CHO cells or COS cells.
[0050] In a preferred embodiment of the present invention, the gene encoding the fusion protein is expressed in HEK 293-F cell expression system. HEK293 is a stable cell line obtained after transfection of human embryonic kidney cells by adenovirus Ad5. HEK293-F is a derivative cell line of HEK293, which has easy transfection, high expression, natural glycosylation modification, allows correct protein folding and related translation Post-modification and other advantages.
[0051] In some embodimen...
Embodiment 1
[0065] Embodiment 1 encodes the fusion protein sequence of porcine pseudorabies virus
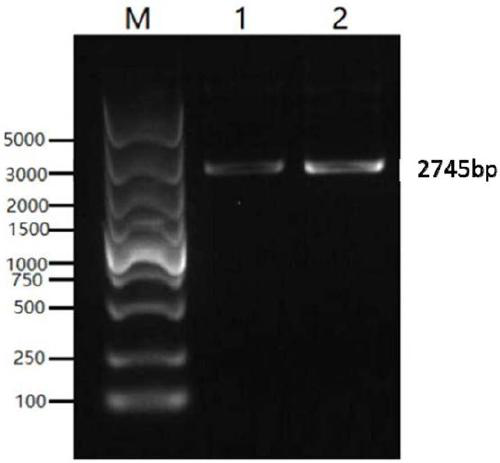
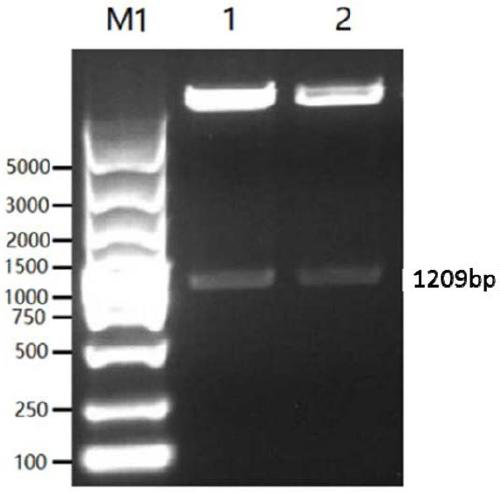
[0066] The genes of the classic strain (SC strain) and the current epidemic strain (JS strain) were selected from Genebank as the research objects. Through comparative analysis, the dominant epitope was selected as the component of the vaccine antigen. According to the codons of HEK 293-F cells partial tropism, and this sequence is carried out codon optimization and modification, obtains the nucleotide sequence shown in SEQ ID NO.1 and SEQ ID NO.2, the agarose gel electrophoresis figure of PCR enrichment gB sequence is as follows figure 1 As shown, the agarose gel electrophoresis image of the PCR-enriched gD sequence is as follows figure 2 shown. The middle of the gB and gD sequences is connected with the sequence shown in SEQ ID NO.3 to achieve the purpose of further improving the broad spectrum of the fusion protein antigen and increasing the expression of the antigen.
Embodiment 2
[0067] The construction of the recombinant vector of embodiment 2 expression fusion protein
[0068] The sequence encoding the fusion protein synthesized above was cloned into the eukaryotic transfer vector pcDNA3.1 by inserting Mlu I and Hind III sites. Use T4 DNA ligase to ligate overnight at 16°C to obtain ligation products, transform Escherichia coli competent DH5α, spread on LB plates containing ampicillin, culture overnight at 37°C, and pick positive colonies on LB plates containing ampicillin Cultivate in culture medium and extract plasmid. The correct recombinant plasmid was obtained through PCR, double enzyme digestion and sequencing verification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com