Synthesis method of amikacin
A technology of amikacin and kanamycin, which is applied in the field of medicine, can solve the problems of increasing the amount of reaction solvent used, increasing the production cost, and easy volatilization of the solvent, so as to simplify the production equipment, facilitate the production operation, and reduce the use of solvents Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The present embodiment provides a method for synthesizing amikacin, comprising the steps of:
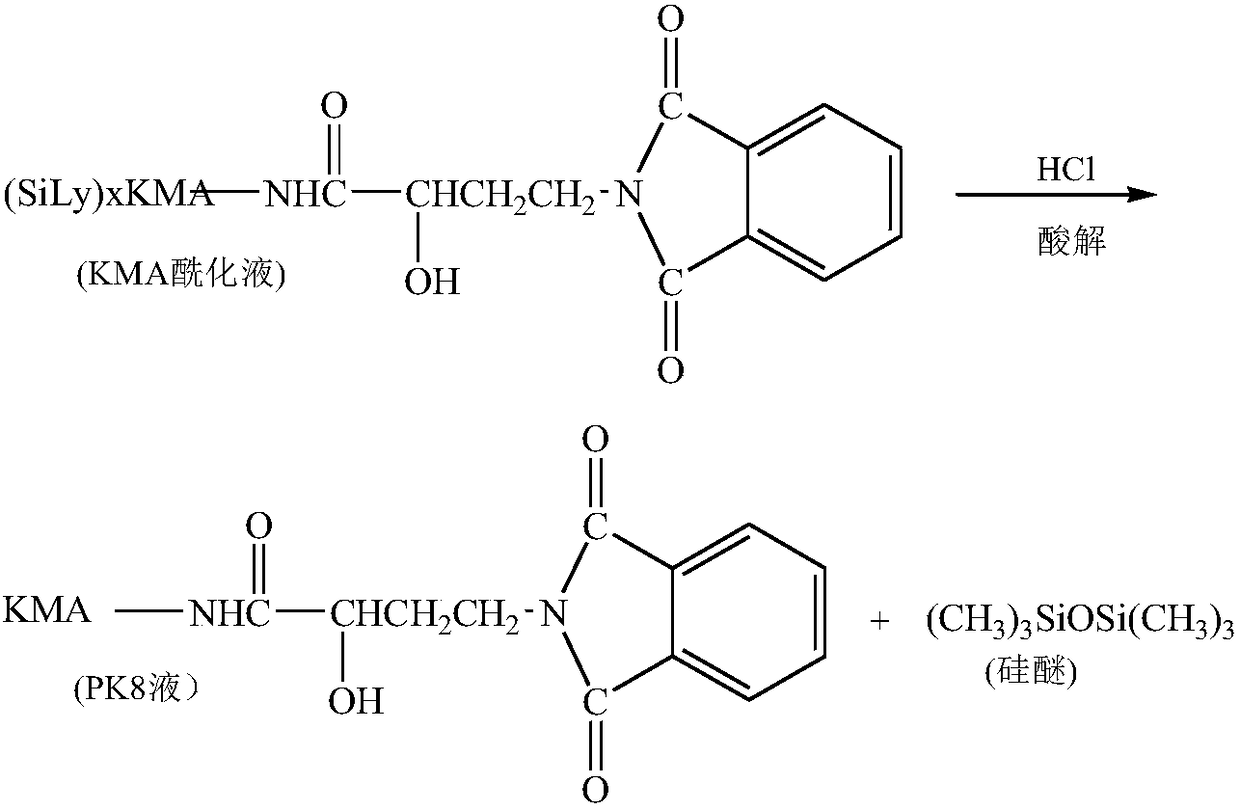
[0059] 1) Catalyzed silylation reaction: Put 600mL tetrahydrofuran into the silanization reaction bottle, add 0.1 billion kanamycin (KMA), add 190mL hexamethyldisilazane (HMDS), add catalyst triethylamine salt Salt 0.5g, start stirring, heat up to reflux, reflux reaction at 60-70°C for 130 minutes, vacuum distillation, vacuum degree ≤ -0.085Mpa, temperature 75-80°C until no solvent flows out, silanized product;
[0060] 2) Acylation reaction: add 1000mL tetrahydrofuran to the silylation product, start stirring, add 55g γ-N-phthalimide-α-hydroxybutyric acid (PHBA), add 48g N,N-dicyclohexyl Carbodiimide, control the temperature of the reactants at 25°C, and react for 120 minutes.
[0061] 3) Acidolysis reaction: After the acylation reaction is completed, start stirring, add 350ml of 4.0mol / L hydrochloric acid, make the pH of the feed solution 3.0, and acidify at room temperatur...
Embodiment 2
[0066] The present embodiment provides a method for synthesizing amikacin, comprising the steps of:
[0067] 1) Catalyzed silylation reaction: Put 600mL tetrahydrofuran into the silanization reaction bottle, add 0.1 billion kanamycin (KMA), add 200mL hexamethyldisilazane (HMDS), add catalyst diethylamine salt Salt 0.3g, start stirring, heat up to reflux, reflux at 60-70°C for 110 minutes, distill under reduced pressure, vacuum degree ≤ -0.085Mpa, temperature 75-80°C until no solvent flows out, and the silanized product;
[0068] 2) Acylation reaction: add 1000mL tetrahydrofuran to the silylation product, start stirring, add 60g γ-N-phthalimide-α-hydroxybutyric acid (PHBA), add 52g N,N-dicyclohexyl Carbodiimide, control the temperature of the reactants at 30°C, and react for 100 minutes.
[0069] 3) Acidolysis reaction: After the acylation reaction is completed, start stirring, add 360ml of 4.0mol / L hydrochloric acid, make the pH of the feed solution 3.5, and acidolyze at room...
Embodiment 3
[0074] This embodiment provides a method for synthesizing amikacin. The only difference from Example 1 is that in step 1), "catalyst triethylamine hydrochloride" is replaced by "triethylamine sulfate";
[0075] Obtain amikacin synthetic solution 1780ml, the content of amikacin is 2.1% (g / ml) and the synthesis yield of kanamycin A is 31.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com