Cytochrome P450, nicotinamide adenine dinucleotide-cytochrome P450 reductase and application thereof
A technology of nicotinamide adenine and cytochrome, applied in the fields of biotechnology and microbiology, can solve the problems of high cost and limited yield of Ganoderma lucidum triterpenes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0124] Example 1, Cloning of Cytochrome P450 and Nicotinamide Adenine Dinucleotide (NADPH)-cytochrome P450 Reductase
[0125] The six primers synthesized respectively have the nucleotide sequences of SEQ ID NO:4, SEQ ID NO:5, SEQ ID NO:6, SEQ ID NO:7, SEQ ID NO:8 and SEQ ID NO:9 in the sequence listing.
[0126] Ganoderma lucidum RNA was extracted and reverse-transcribed to obtain ganoderma lucidum cDNA. The cDNA was used as a template for PCR amplification, and the above three pairs of primers SEQ ID NO:4 / 5, SEQ ID NO:6 / 7 and SEQ ID NO:8 / 9 were used for PCR amplification respectively. The high-fidelity PrimeSTAR DNA polymerase from Treasure Bioengineering Co., Ltd. was selected as the DNA polymerase. PCR products were detected by agarose gel electrophoresis ( figure 1 ). Under UV irradiation, the target DNA band is excised. Then, Axygen Gel Extraction Kit (AEYGEN Company) was used to recover DNA from the agarose gel, which was the amplified DNA fragment. Ligate this DNA...
Embodiment 2
[0130] Example 2, Yeast Recombinant Expression Vector Construction of Cytochrome P450 Gene GPO1 and GPO2
[0131]The synthetic primers respectively have SEQ ID NO:10, SEQ ID NO:11, SEQ ID NO:12, SEQ ID NO:13, SEQ ID NO:14, SEQ ID NO:15, SEQ ID NO:24 and SEQ ID in the sequence listing Nucleotide sequence of NO:25.
[0132] Using the genome of Saccharomyces cerevisiae CEN.PK113-3C (purchased from Euroscarf) as a template, the Trp expression element dTrp-1 (that is, dTrp, whose 5' and 3' ends are both amplified with primers SEQ ID NO:24 and SEQ ID NO:25) Contains sequences homologous to pESC-His). The PCR product was recovered from the agarose gel with the AxyPrep DNA Gel Extraction Kit from AXYGEN. Plasmid pESC-His (purchased from Agilent, USA) was digested with Nde I and Pst I from Fermentas, and DNA fragments were recovered from the agarose gel with AxyPrep DNA Gel Extraction Kit from XYGEN. The DNA fragment and the digested pESC-His plasmid were transformed into Saccharomy...
Embodiment 3
[0136] Example 3, Expression of Cytochrome P450 Genes GPO1 and GPO2 in Saccharomyces cerevisiae
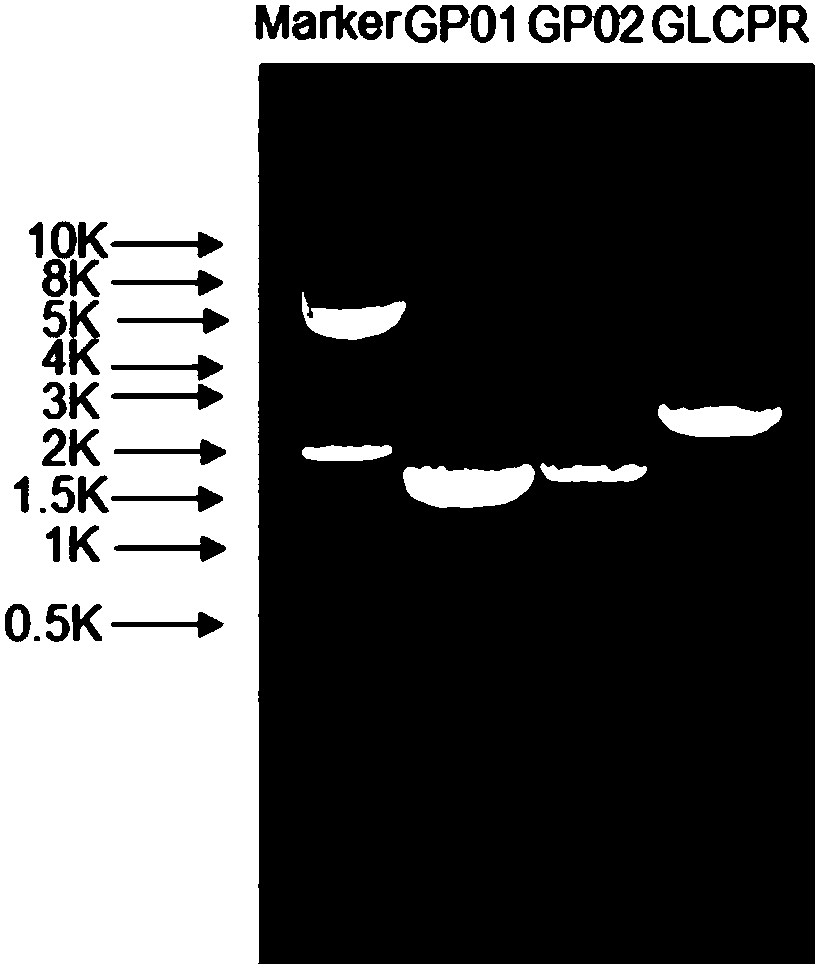
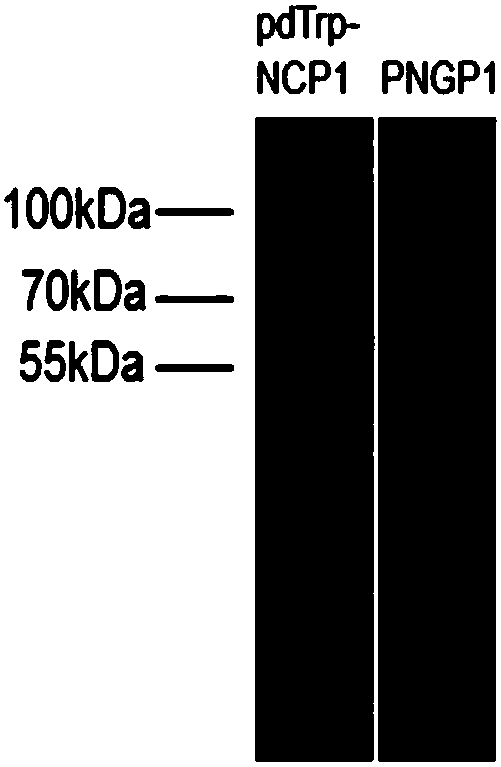
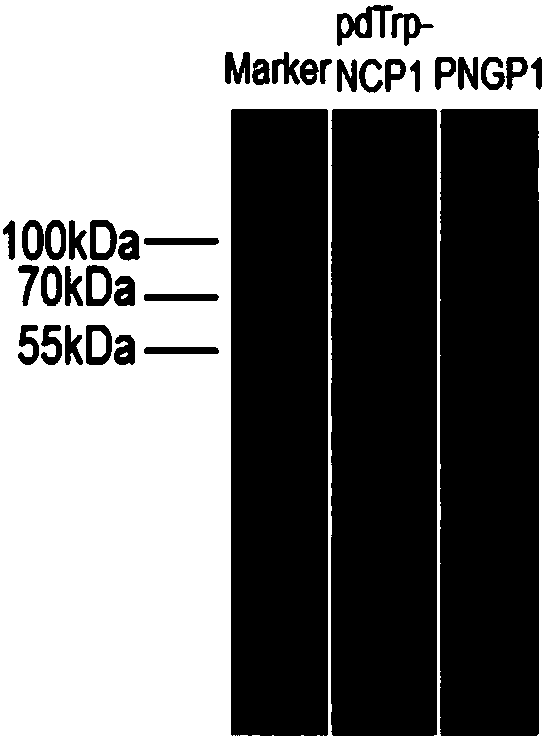
[0137] Preparation of SCO medium: 0.67% (w / v) parent amino acid-free basic nitrogen source, 2% (w / v) glucose. Inoculate PNGP1 and PNGP1 in SCO medium, culture at 250 rpm at 30°C to reach the stationary phase. Prepare YPD medium: 1% (w / v) yeast extract, 2% (w / v) bacto-peptone, 2% (w / v) glucose, collect the bacteria by centrifugation, resuspend in rich YPD medium, 30 Cultivate at 250rpm for 24h. Glucose was replaced by galactose to induce protein expression. Bacteria were collected by centrifugation, cells were lysed, and microsomes were obtained by ultracentrifugation. Take an appropriate amount of microsomes for SDS-PAGE electrophoresis detection. Compared with the recombinants transfected with the pdTrp-NCP1 control vector, PNGP1 and PNGP2 recombinants have no obvious band characteristics, such as image 3 with Figure 5 . Use anti-6×His TagWestern Blot to detect expression...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com