Method for synthesis of (S)-rivastigmine through reductive amination
A synthesis method and compound technology, which are applied in the field of chiral drug synthesis in pharmaceutical and chemical industry, can solve the problems of unsatisfactory yield, expensive starting materials, harsh reaction conditions, etc., and achieve short synthesis steps and good industrial application. Prospect, effect of fewer reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0131] (1) Synthesis of N-methylethyl (3-acetylphenyl) carbamate (compound 3)
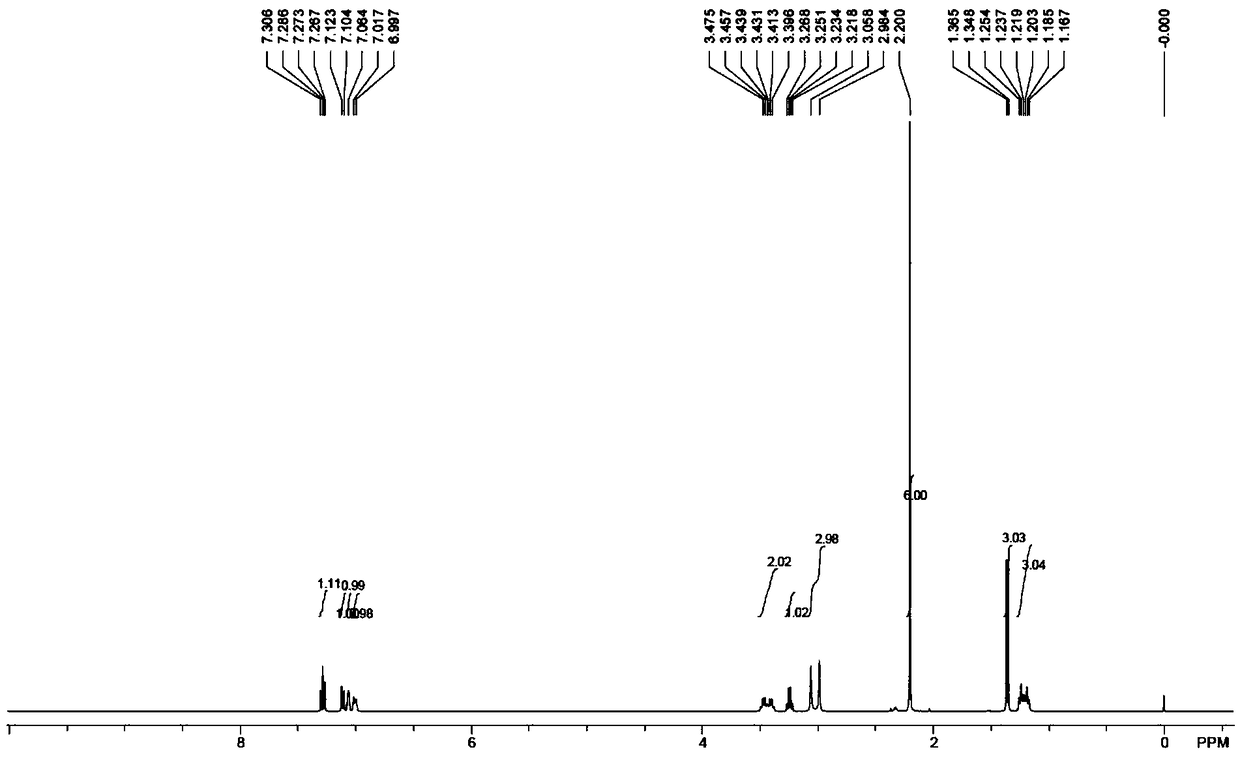
[0132] 3-Hydroxyacetophenone (5.1g, 38mmol) and methylethylcarbamoyl chloride (7.2g, 58mmol) were added to acetone (100mL) in turn. After the reaction solution was dissolved, potassium carbonate (10.4g, 76mmol) was added. After the addition, the temperature was raised to 60°C, and the reaction was stirred for 4 hours. After the reaction was completed, the temperature was lowered to room temperature, filtered, and the filter cake was washed with acetone (20 mL). The filtrate was concentrated under reduced pressure to obtain a light yellow oily compound 3 (with 3-hydroxyacetophenone Total, the yield is 100%). 1 H-NMR (500MHz, Chloroform-d): δ7.83(d,J=7.7Hz,1H), 7.74(s,1H), 7.50(t,J=7.9Hz,1H), 7.39(s,1H) , 3.50(dq,J=35.8,7.1Hz,2H), 3.09(d,J=42.1Hz,3H), 2.64(s,3H), 1.28(dt,J=29.2,7.1Hz,3H)
[0133] (2) Synthesis of (Compound 4)
[0134] Compound 3 (221mg, 1mmol) and benzhydrylamine (238mg, 1.3mmol) were add...
Embodiment 2
[0140] (1) Synthesis of N-methylethyl (3-acetylphenyl) carbamate (compound 3)
[0141] 3-Hydroxyacetophenone (5.1g, 38mmol) and methylethylcarbamoyl chloride (7.2g, 58mmol) were added to ethyl acetate (80mL) in turn. After the reaction solution was dissolved, triethylamine (4.04g, 40mmol), after the addition, the temperature was raised to 40°C, stirred for 7 hours, after the reaction was completed, cooled to room temperature, filtered, and the filter cake was washed with ethyl acetate (25mL), and the filtrate was concentrated under reduced pressure to obtain a light yellow oily compound 3 (with 3 -Based on hydroxyacetophenone, the yield is 100%).
[0142] (2) Synthesis of (Compound 4)
[0143] Compound 3 (221mg, 1mmol) and benzhydrylamine (366mg, 2.0mmol) were sequentially added to stirring acetone (10mL). After the reaction solution was dissolved, formic acid (0.8mmol) and tetraethyl titanate (0.5mmol) were added. , Then add Ir catalyst (22.1mg) and molecular sieves, place the rea...
Embodiment 3
[0149] (1) Synthesis of N-methylethyl (3-acetylphenyl) carbamate (compound 3)
[0150] 3-Hydroxyacetophenone (5.1g, 38mmol) and methylethylcarbamoyl chloride (7.2g, 58mmol) were added to toluene (150mL) in sequence. After the reaction solution was dissolved, diisopropylethylamine (5.17 g, 40mmol), after the addition, the temperature was raised to 80°C, the reaction was stirred for 2.5 hours, the reaction was completed, the temperature was lowered to room temperature, filtered, the filter cake was washed with toluene (30mL), the filtrate was concentrated under reduced pressure to obtain a light yellow oily compound 3 (with 3 -Based on hydroxyacetophenone, the yield is 96%).
[0151] (2) Synthesis of (Compound 4)
[0152] Compound 3 (221mg, 1mmol) and benzhydrylamine (238mg, 1.3mmol) were added to the stirring dichloromethane (15mL) in turn, the reaction solution was dissolved and added p-nitrobenzoic acid (0.2mmol) and titanate Isobutyl ester (0.5mmol), then add Ir catalyst (22.1mg)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com