Application of ethyl lauroyl arginate derivative as feed additive
A technology of lauroyl arginine ethyl ester and feed additives, which is applied in the field of ion-pair compounds of lauroyl arginine ethyl ester, can solve the problems of untaught and unstudied antibacterial effect of LAE alone, so as to improve antibacterial activity, Improve bioavailability and improve absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1: The preparation method of the ion-pair compound synthesized by lauroyl arginine ethyl ester hydrochloride and nicotinic acid
[0074] Dissolve 2.0 g of sodium nicotinate (purchased from TCI (Shanghai) Chemical Industry Development Co., Ltd.) in 50 mL of water to prepare sodium nicotinic acid salt solution (A); dilute 6.8 g of ethyl lauroyl arginate hydrochloride Dissolve in 40mL of water, heat to 90°C until ethyl lauroyl arginine hydrochloride is completely dissolved to make ethyl lauroyl arginine hydrochloride aqueous solution (B); Slowly add the saline solution (A) into the aqueous solution of lauroyl arginine ethyl ester hydrochloride (B), stir continuously, react for 2 hours, cool to room temperature, filter, wash the precipitate fully with pure water, and dry the precipitate under vacuum at 60°C. That is, 7.6 g of the nicotinic acid ion pair compound was obtained.
Embodiment 2
[0075] Embodiment two lauroyl arginine ethyl ester nicotinic acid ion pair compound molecular formula, the analysis of molecular weight
[0076] by mass spectrometry, 1 H-NMR, 13 The molecular formula of the compound obtained by C-NMR spectral analysis is:
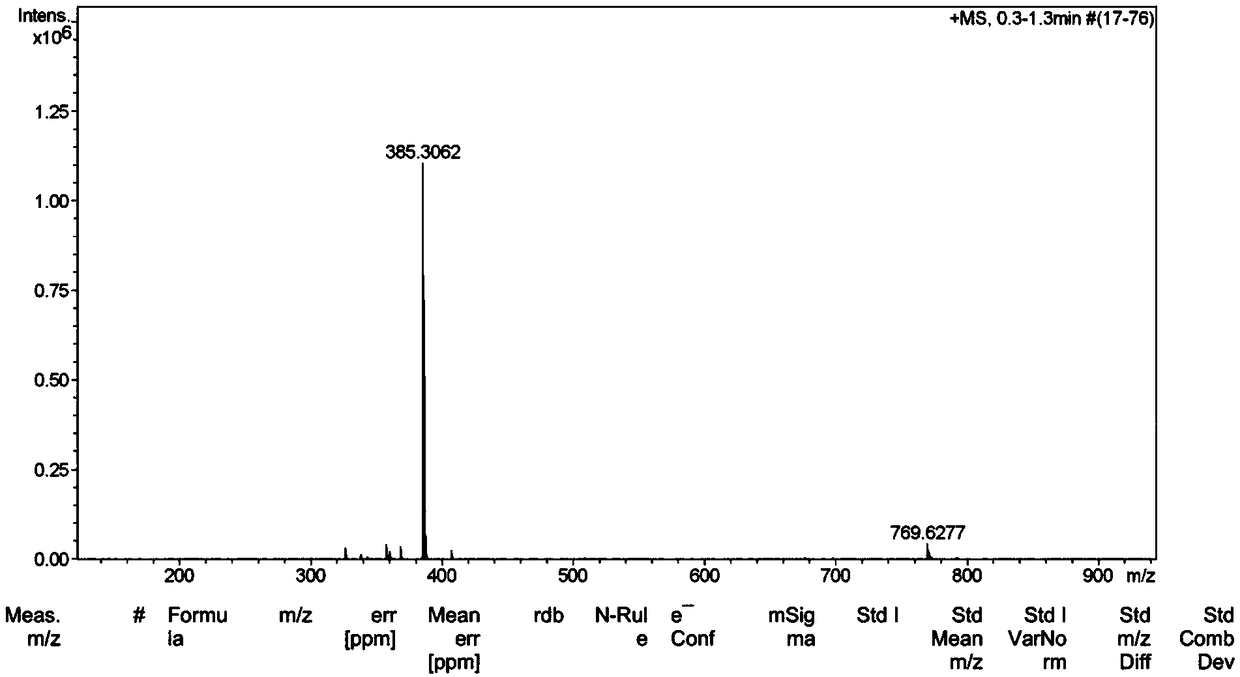
[0077] 1. Mass spectrometry (ESI) analysis
[0078] Cation B + Molecular ion peak at m / z=385.3, see figure 1 ;
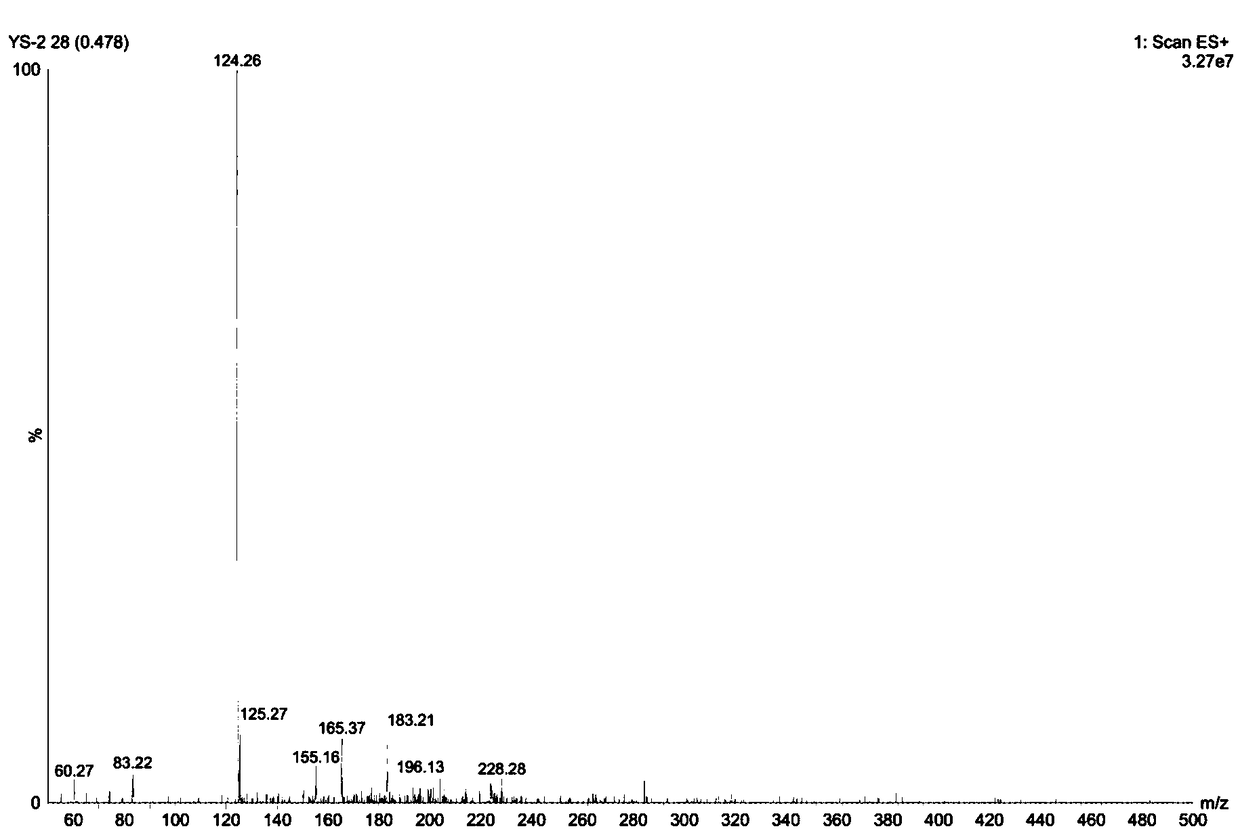
[0079] The ESI+ detected by mass spectrometry is 124.2, see figure 2 . Then ESI- is 122.2, namely anion A - The molecular ion peak has m / z = 122.2.
[0080] The theoretically calculated value of the cation in the nicotinic acid ion-pair compound is 507.4, and the measured value is consistent with the theoretical value.
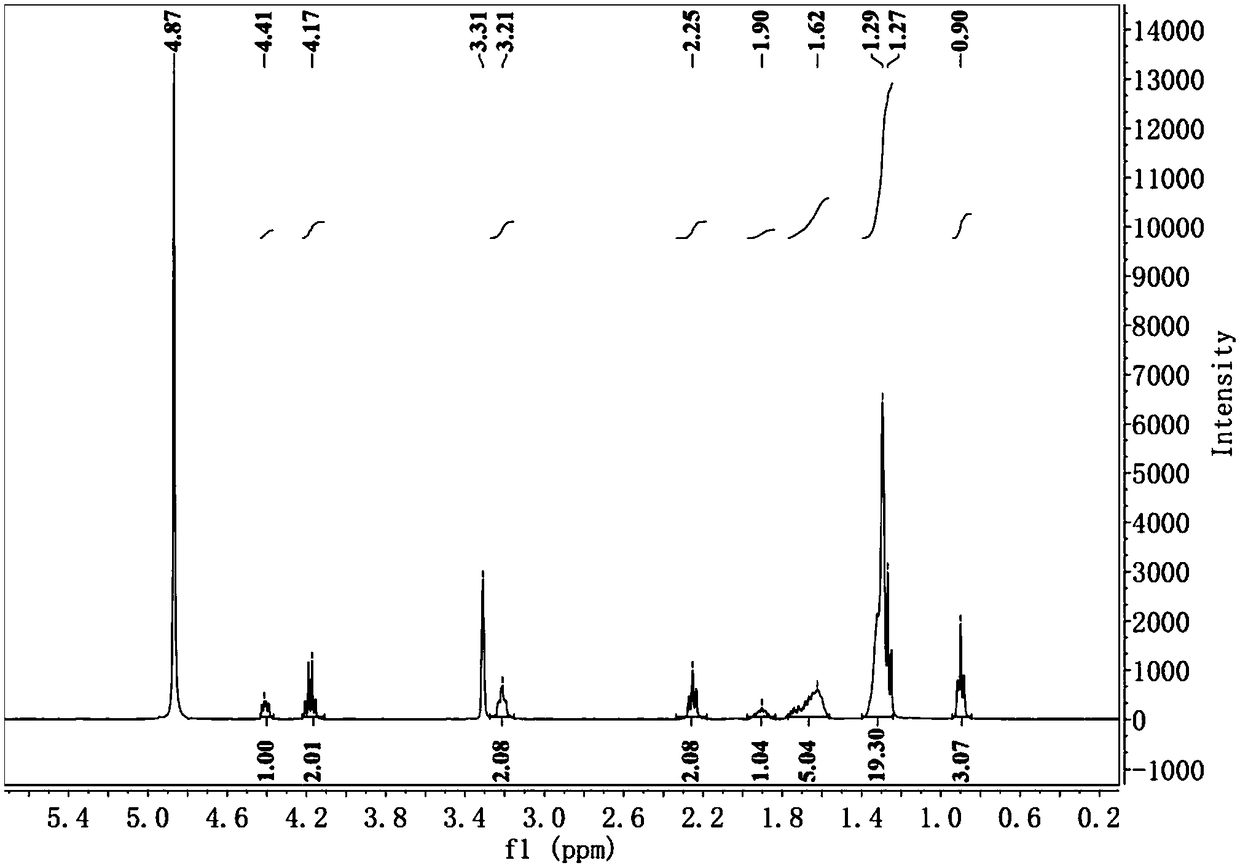
[0081] 2. NMR analysis
[0082] Ethyl lauroyl arginate hydrochloride (see image 3 ), niacin 1 H-NMR (see Figure 4 ) and ion-pair compounds 1 H-NMR (see Figure 5 )Compared. Since the LAE ion-pair compound has little change in the peak shape and chemical shift of lauroyl argini...
Embodiment 3
[0083] Example 3: Preparation method of ethyl lauroyl arginine hydrochloride and tartaric acid synthetic ion pair compound
[0084] Dissolve 2.0 g of tartaric acid (purchased from TCI (Shanghai) Chemical Industry Development Co., Ltd.) in 50 mL of methanol, add an equivalent amount of NaOH, stir at room temperature until a white solid precipitates, filter with suction and wash three times with 30 mL of methanol to obtain sodium tartrate . Sodium tartrate salt was dissolved in 50mL of water to make sodium tartrate aqueous solution (A); Dissolve 5.6g of ethyl lauroyl arginine hydrochloride in 40mL of water and heat to 90°C until ethyl lauroyl arginine salt The acid salt was completely dissolved to make ethyl lauroyl arginine hydrochloride aqueous solution (B); at 90°C, sodium tartrate aqueous solution (A) was slowly added to ethyl lauroyl arginine hydrochloride aqueous solution ( In B), stir continuously, react for 2 hours, cool to room temperature, filter, wash the precipitate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com