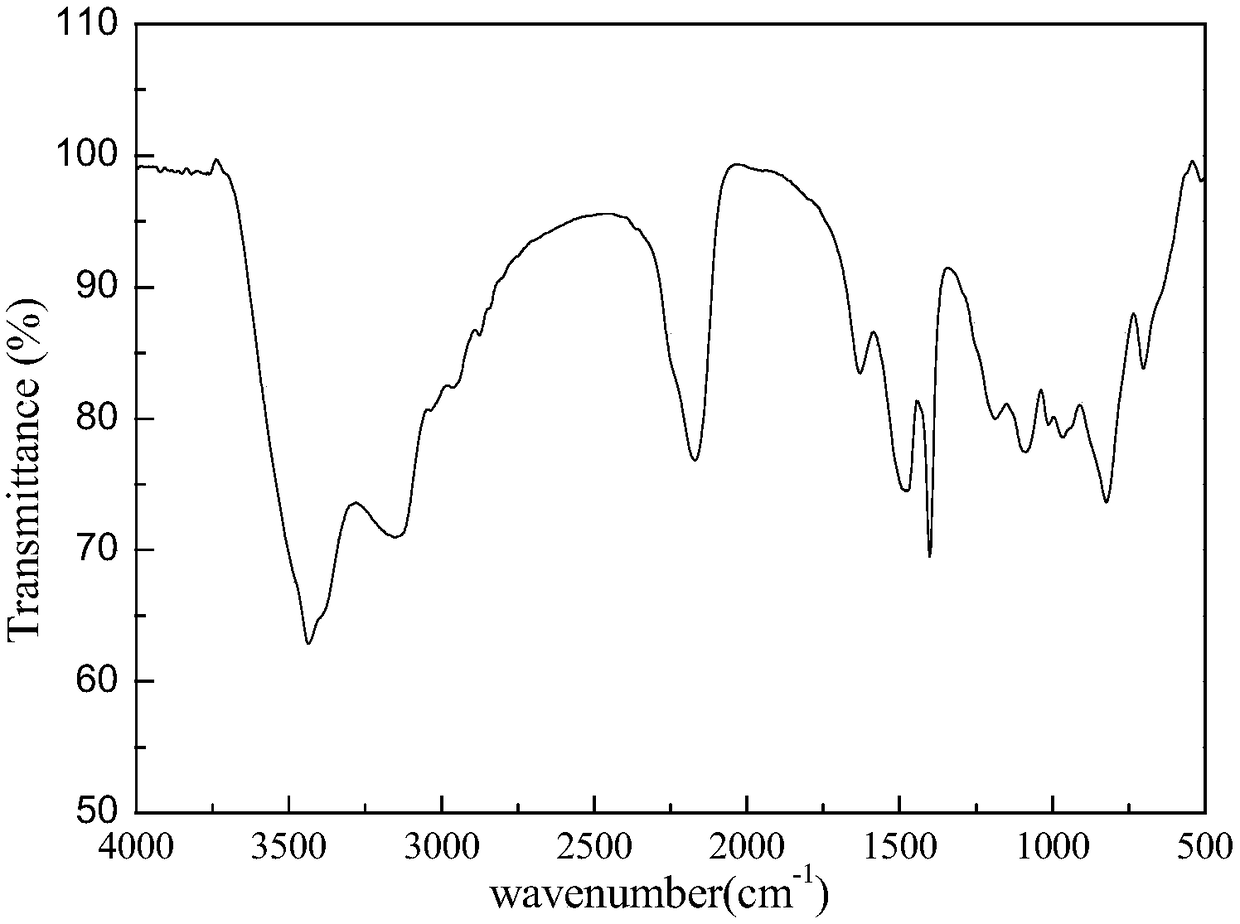
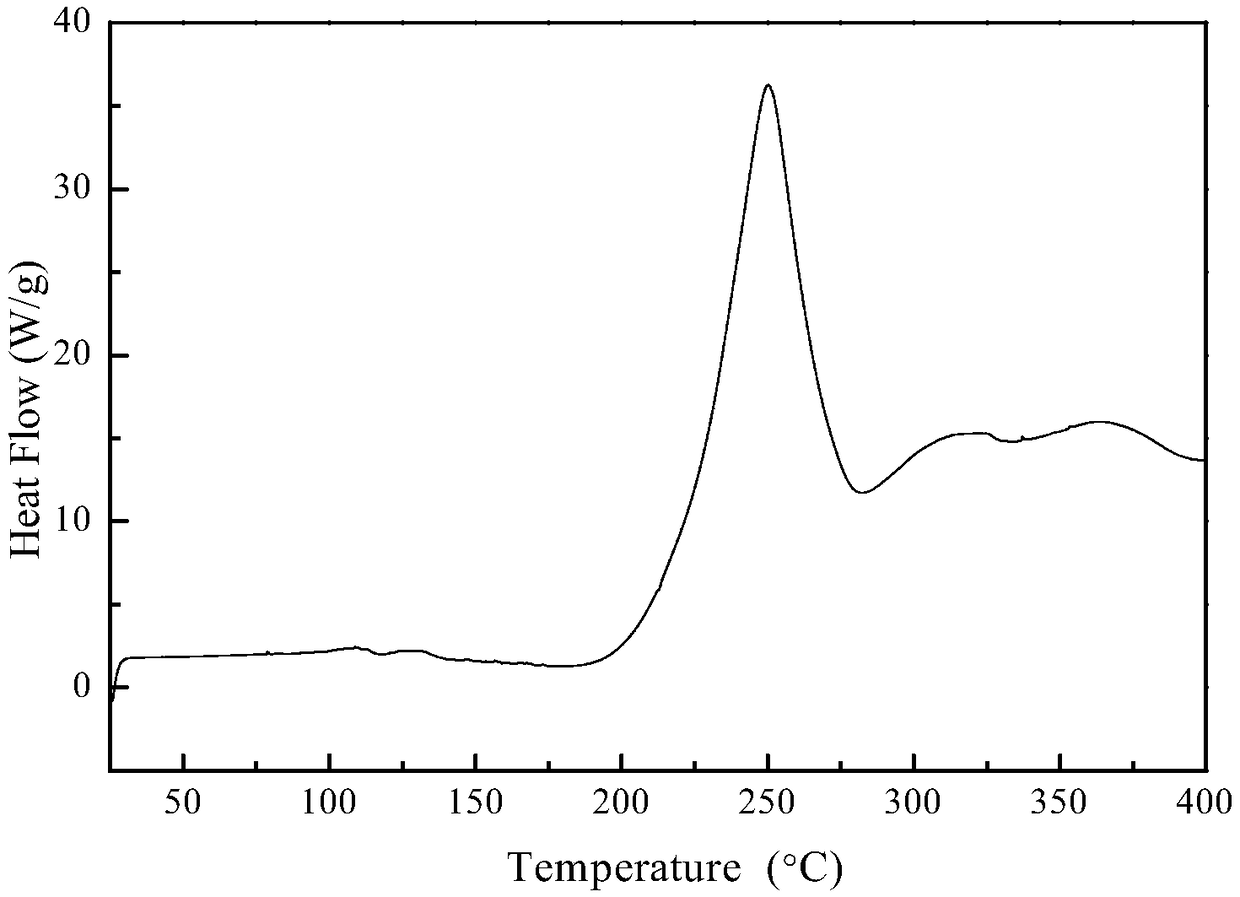
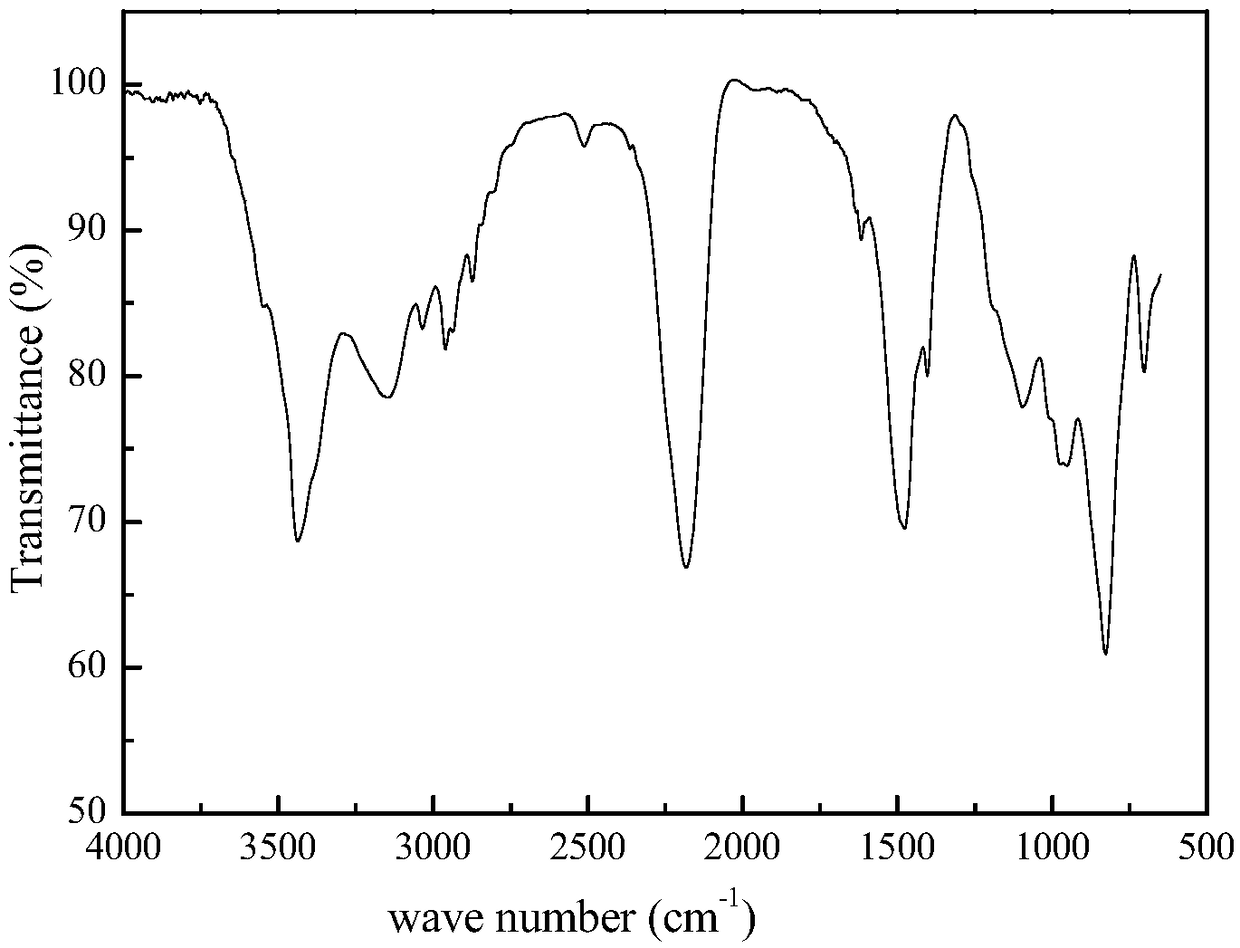
Silicon-boron-azane ceramic precursor polymer containing borazine and preparation method and application of polymer
The technology of a ceramic precursor and borazane is applied in the field of preparation of the precursor polymer, which can solve the problems of low ceramization yield, poor process performance, affecting the performance of ceramic matrix composite materials, etc., and achieves excellent process performance, The effect of high ceramic yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Precursor PBSZ-1
[0038] In a 500ml four-necked flask equipped with a nitrogen conduit, a stirrer, a thermometer and a condenser tube, after several cycles of dehumidification and deoxygenation by vacuuming and passing nitrogen, add NH 4 Cl powder 32g (0.30mol) and toluene 300ml, and vigorously stirred to form a suspension, heated to 110 ° C, slowly into the four-necked flask BCl 3 Gas, and insulation reaction 10h. Then cool down to room temperature, adopt shleck technique to suction filter under nitrogen protection, the filtrate is distilled off under reduced pressure to remove the solvent, and then sublimated to obtain colorless needle-shaped crystal trichloroborazine (TCB).
[0039] In the 2000ml four-neck flask equipped with nitrogen conduit, stirrer, thermometer and condensing tube, after vacuumizing, passing nitrogen several times to circulate dehumidification and deoxygenation, under the protection of nitrogen, add trichloroborazine 9g and Dichlorome...
Embodiment 2
[0043] Example 2 Precursor PBSZ-2
[0044] In a 500ml four-necked flask equipped with a nitrogen conduit, a stirrer, a thermometer, and a condenser tube, after several cycles of dehumidification and oxygen removal by vacuuming and nitrogen, add BCl 3 (0.09mol) of toluene solution, cooled to -10°C with salt ice, slowly dropwise added a mixture of 18.22g (0.18mol) of triethylamine and toluene (20mL) to it, and then added olefin Propylamine 5.14g (0.09mol) and toluene (10mL), then the system was slowly raised to 110°C for 6h, then cooled to room temperature. The shleck technique was used for suction filtration under the protection of nitrogen, the filtrate was transferred to a rotary evaporator, and the solvent was distilled off under reduced pressure to obtain a colorless and transparent liquid triallyltrichloroborazine (TV-TCB).
[0045] After checking the airtightness of the reaction system of the 2000ml four-necked flask equipped with a PTFE stirring rod, a thermometer, a sy...
Embodiment 3
[0047] Example 3 Precursor PBSZ-3
[0048] After checking the airtightness of the reaction system of the 2000ml four-necked flask equipped with a PTFE stirring rod, a thermometer, a syringe and a nitrogen gas guide tube, bake and vacuumize repeatedly, and then add the reaction system to it sequentially under the protection of nitrogen gas. Trichloroborazine 2.18g and dichloromethane 1400ml, start stirring, make trichloroborazine dissolve and disperse in dichloromethane, then, at normal temperature, slowly add 1mol / L of methylmagnesium bromide Grignard reagent 12ml, after the dropwise addition, the reaction system was reacted at room temperature for 3 hours to obtain a white solution of methyl-substituted borazine. Place the four-neck flask in a low-temperature bath at -10°C, then add 40 g of dichlorodihydrosilane to it, and finally feed ammonia gas into the reaction system to start the reaction until the reaction system is alkaline, stop the reaction, and then pump Filter, r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com