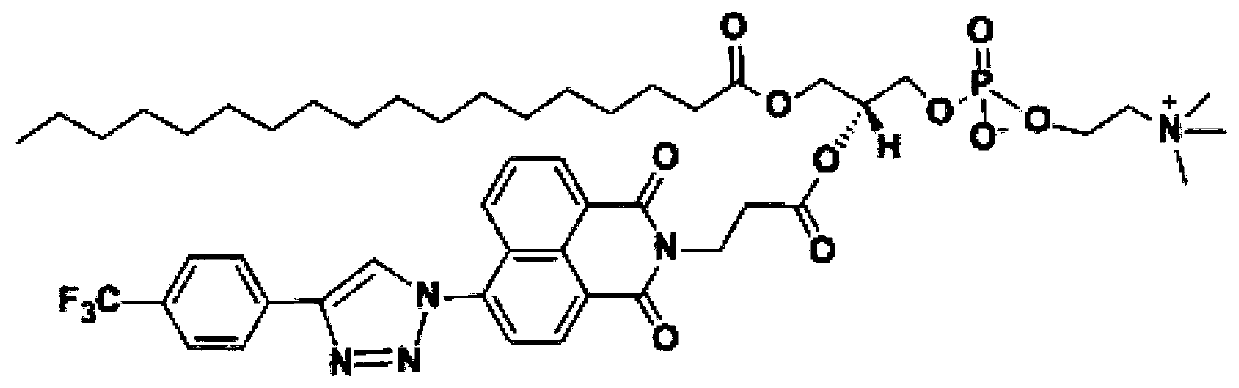
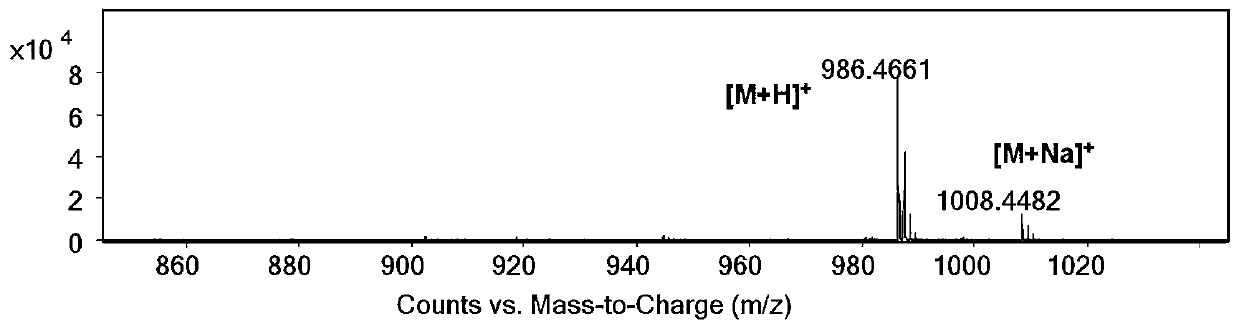
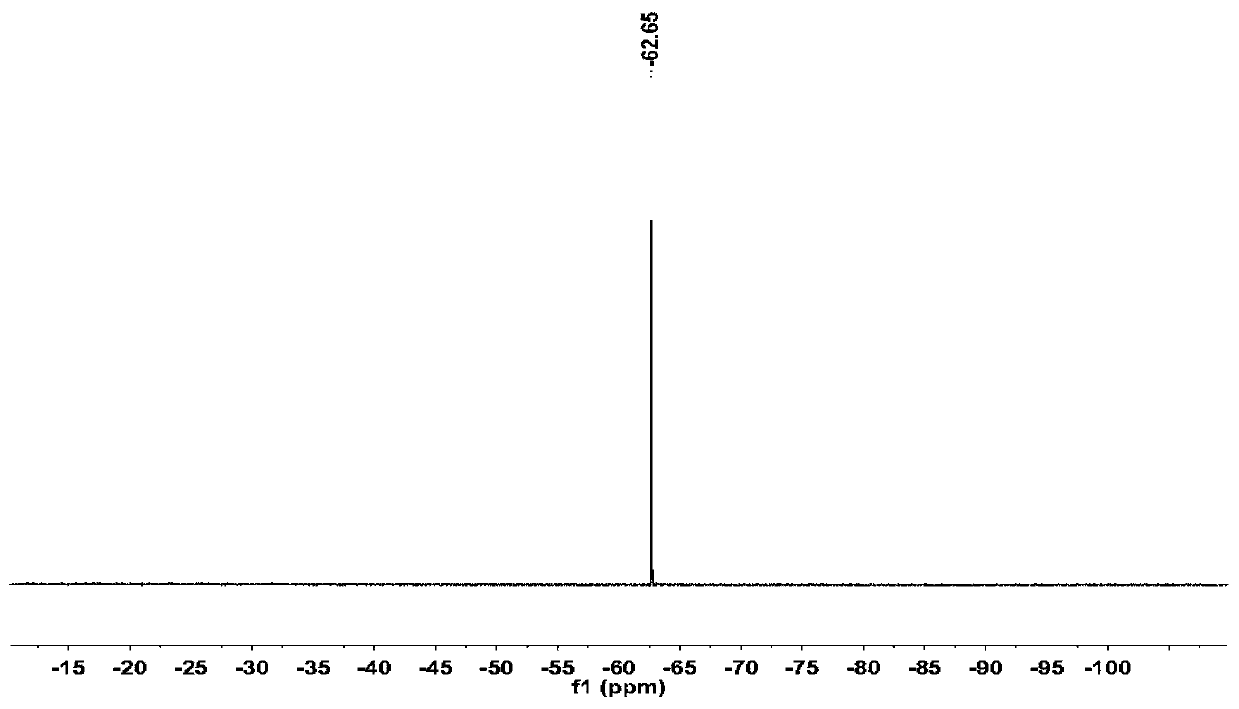
A kind of fluorinated fluorescent phospholipid and its synthesis method and application
A technology of fluorescent phospholipid and synthesis method, which is applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve the problem that contrast agents cannot be effectively detected by MRI, affect the loading of other functional molecules, fluorine-based The problems of low encapsulation efficiency of compounds can increase the stability and application value, increase the water solubility and biocompatibility, and improve the poor water solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] A synthetic method for fluorinated fluorescent phospholipids, comprising the steps of:
[0053] (1) Dissolve 1g (3.62mM) of 4-bromo-1,8-naphthalene anhydride in 30ml of ethanol, add 323mg (3.62mM) of alanine, then reflux at 80°C for 8h, and monitor the reaction progress by thin-layer chromatography. After the reaction solution changed from gray to brown, the reaction was stopped, and the reaction product was subjected to vacuum filtration, and the solid obtained by suction filtration was redissolved in 30ml of ethanol, and then subjected to suction filtration, and the obtained solid was vacuum-dried to obtain a brown solid compound 1 with a yield of about 90% %, no need for further purification, directly used in the next step; wherein, the structural formula of compound 1 is:
[0054]
[0055] As can be seen from the above, the reaction formula of this step is as follows:
[0056]
[0057] (2) Dissolve 1g (2.88mM) of compound 1 prepared in step (1) in 20ml of DMF...
Embodiment 2
[0071] The specific process of labeling fluorinated thermosensitive liposomes with fluorinated fluorescent phospholipids described in Example 1 is:
[0072] Dissolve 14.68mg DPPC, 1.57mg MPPC, 2.49mg PEG2000-DSPE, 2.19mg F-PC (90:10:4:10, molar ratio) in 5ml chloroform, remove the organic solvent by rotary evaporation, and make the phospholipids form a film. Vacuum dry overnight. Then add 4ml of 3.75mg DOX (1:0.2, mass ratio of phospholipid to drug) aqueous solution, ultrasonicate at 60°C for 10min, and extrude 10 times with a 200nm filter membrane. The prepared liposome was dialyzed, the molecular weight cut-off of the dialysis bag was 10KD, the solvent was deionized water, and the dialyzed for 12h. The liquid in the dialysis bag is freeze-dried to obtain the freeze-dried powder of the liposome.
Embodiment 3
[0074] Example 2 Application of fluorinated thermosensitive liposomes labeled with fluorinated fluorescent phospholipids in nuclear magnetic imaging:
[0075] Taking the fluorinated fluorescent phospholipid-labeled fluorinated thermosensitive liposome synthesized in Example 2 as an example to conduct a temperature responsive experiment in an aqueous medium, and to illustrate its use for fluorine imaging under heating conditions.
[0076] Test method: Liposomes are made into different concentrations of aqueous solutions (10, 20, 40, 60, 80 mg / ml, 10% D 2 O+90%H 2 O), 19F NMR at different temperatures (25, 37, 38, 39, 40, 41, 42° C.) were tested with a 500 MHz nuclear magnetic spectrometer. Another 40mg / ml liposome sample was prepared, and its 19F NMR was tested at 42°C at different times (1, 3, 5, 10, 20, 30min) with a 500MHz nuclear magnetic spectrometer, to verify that it was slightly higher than body temperature. temperature responsiveness. Another 20mg / ml liposome sample...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com