Pleuromutilin derivative containing thiazole-picolyl heteroaromatic quaternary ammonium salt side chain as well as preparation method and application of pleuromutilin derivative
A technology of pleuromutilin and picolylmethylaron, which is applied in the field of pleuromutilin derivatives and its preparation, can solve problems such as untreatable, multi-drug resistance, adverse reactions that cannot be widely used, etc., to improve poor water solubility, The effect of excellent antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] (1) Preparation of Intermediate I
[0061]
[0062] Pleuromutilin (757mg, 2mmol) and p-toluenesulfonyl chloride (456mg, 2.4mmol) were dissolved in dichloromethane (80mL), placed in a reactor, TEA (0.8mL, 6mmol) and DMAP (24.4mg, 0.2 mmol), stirred at room temperature for 5 h. The reaction solution was concentrated under reduced pressure, and the concentrated product was washed with saturated aqueous sodium bicarbonate solution (50 mL) to obtain Intermediate I (1012.1 mg, 1.9 mmol) with a yield of 95.0%. The prepared intermediate I was used as the starting material for intermediate II.
[0063] (2) Preparation of Intermediate II
[0064]
[0065] Intermediate I (1012.1 mg, 1.9 mmol) and 2-mercapto-4-(4-pyridyl)thiazole (388 mg, 2 mmol) were dissolved in N,N-dimethylformamide (40 mL) and placed in a reactor , potassium carbonate (524.4 mg, 3.8 mmol) and potassium iodide (31.54 mg, 0.19 mmol) were added, and the reaction was heated at 60 °C for 6 h. After the rea...
Embodiment 2
[0073] Compound 2: 4-(2-((3aR, 4R, 5R, 7S, 8S, 9R, 9aS, 12R)-8-hydroxy-4,7,9,12-tetramethyl-3-oxo-7- Vinyldecahydro-4,9a-propacyclopent[8]cycloalken-5-yl)oxy)-2-oxoethyl)thiazol-4-yl)-1-((5-nitrofuran- Preparation of 2-yl)methyl)pyridyl bromide salt
[0074]
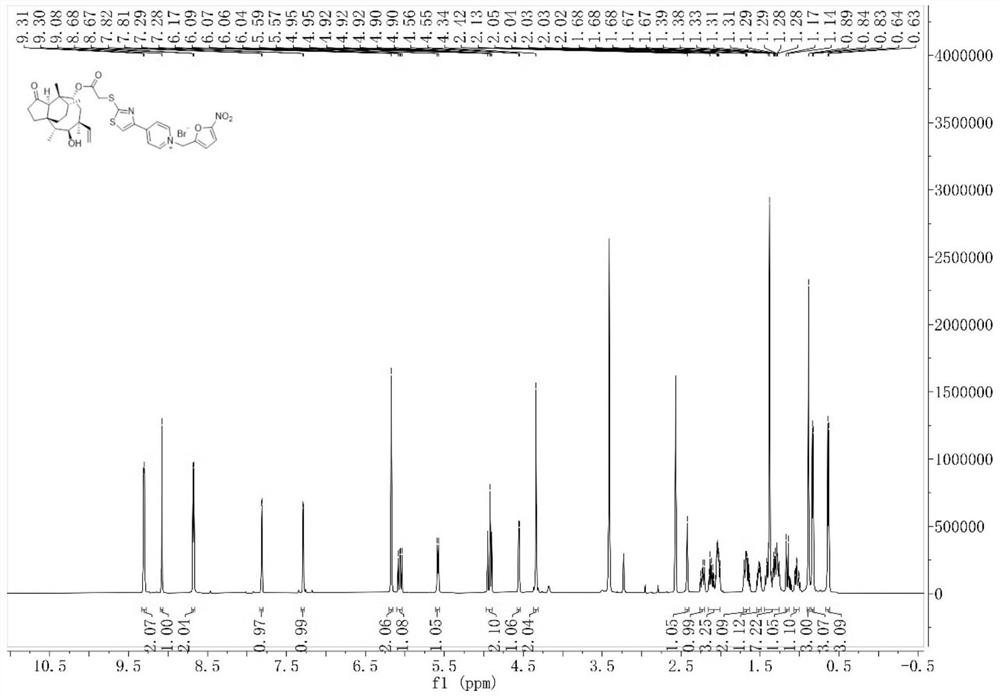
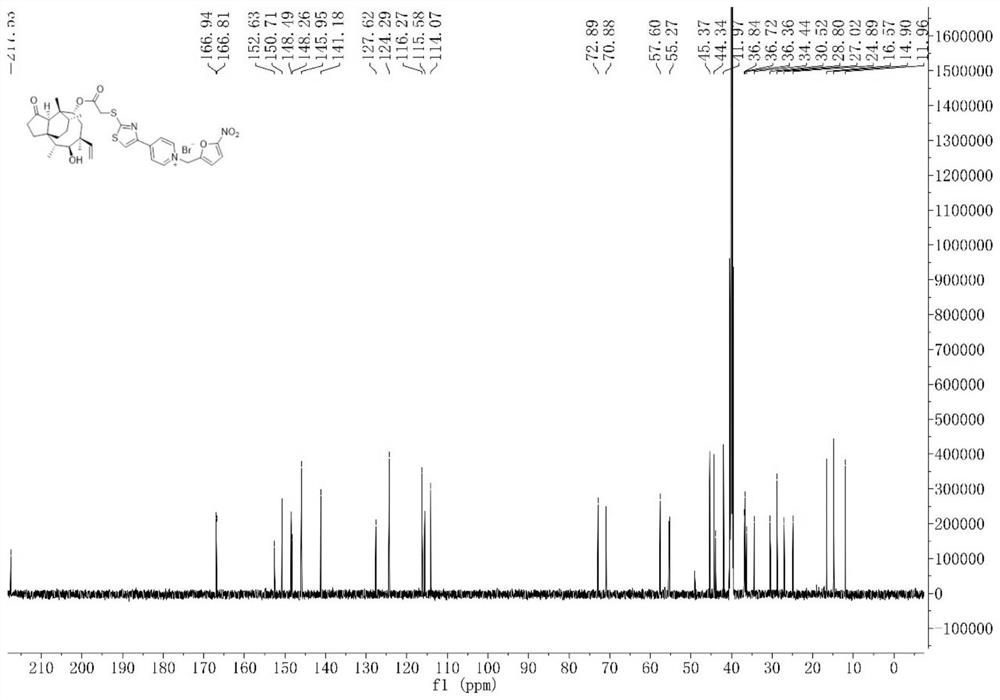
[0075] The preparation process of intermediate I and intermediate II was the same as that of Example 1. Intermediate II (166 mg, 0.3 mmol) and 2-bromomethyl-5-nitrofuran (247.2 mg, 1.2 mmol) were dissolved in dichloromethane ( 10 mL), placed in a reactor, reacted at room temperature for 9 h, concentrated the solvent under reduced pressure, and separated and purified the residue by column chromatography (200-300 mesh silica gel powder was the stationary phase, and the mobile phase was dichloromethane: methanol (V: V) =20:1), dried to give compound 2 (108.68 mg, 0.143 mmol) in 47.7% yield. figure 1 is the hydrogen NMR spectrum of compound 2 in deuterated DMSO, figure 2 C NMR spectrum of compound 2 in deuterated DMSO...
Embodiment 3
[0079]Compound 3: 4-(2-((3aR, 4R, 5R, 7S, 8S, 9R, 9aS, 12R)-8-hydroxy-4,7,9,12-tetramethyl-3-oxo-7- Vinyldecahydro-4,9a-propacyclopent[8]cycloalken-5-yl)oxy)-2-oxoethyl)thiazol-4-yl)-1-(thiophen-3-ylmethyl) ) Preparation of pyridyl bromide salt
[0080]
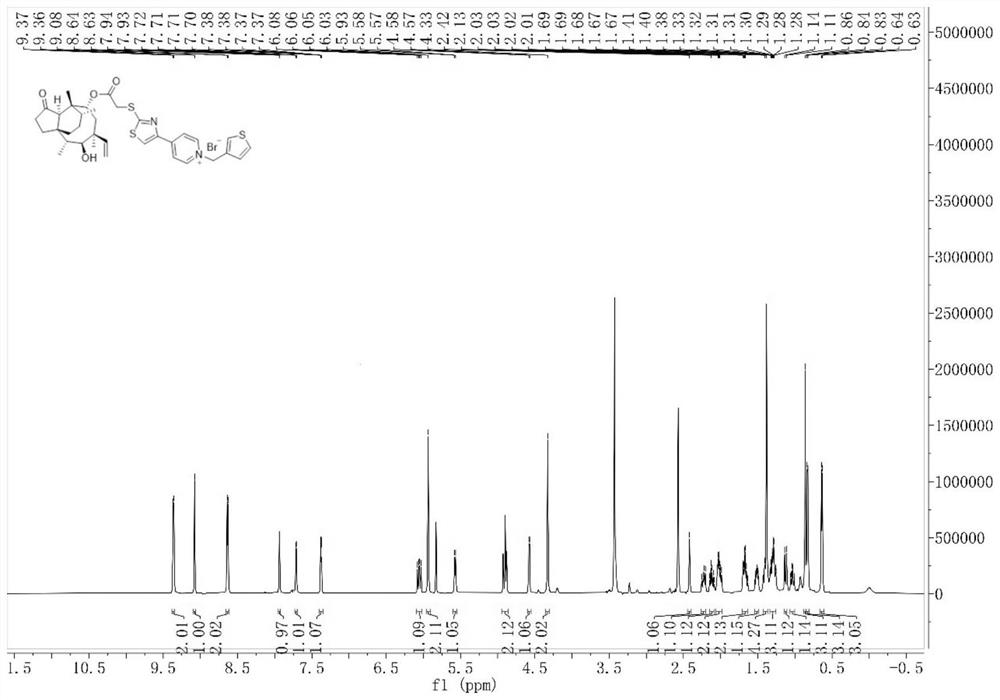
[0081] The preparation process of intermediate I and intermediate II was the same as that of Example 1. Intermediate II (166 mg, 0.3 mmol) and 3-bromomethylthiophene (212.4 mg, 1.2 mmol) were dissolved in acetonitrile (10 mL) and placed in a reactor. , react at room temperature for 8 hours, concentrate the solvent under reduced pressure, and separate and purify the residue by column chromatography (200-300 mesh silica gel powder is the stationary phase, and the mobile phase is dichloromethane:methanol (V:V)=20:1), dry Compound 8 (125.73 mg, 0.172 mmol) was obtained in 57.3% yield.
[0082] 1 H NMR(600MHz, DMSO)δ9.35(d,J=6.5Hz,2H),9.21(s,1H),8.88(d,J=6.4Hz,2H),7.67(d,J=4.2Hz,1H) ), 7.31(d, J=4.2Hz, 1H), 6.21(s, 2H), 6.1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com