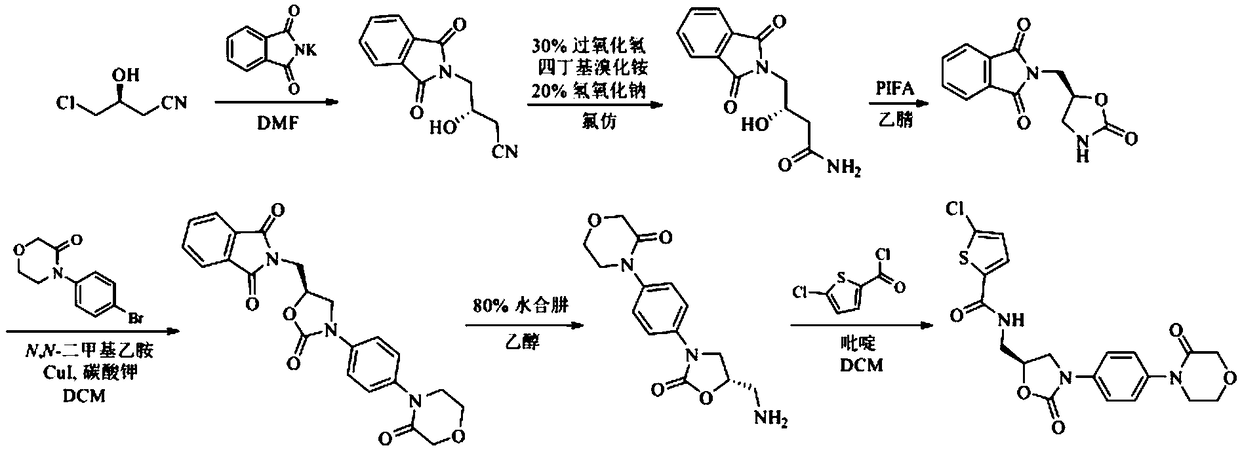
Preparation process for rivaroxaban
A technology for the preparation of rivaroxaban, which is applied in the field of medicinal chemistry, can solve the problems of expensive raw materials, low safety, and low material collection, and achieve the effects of cheap and easy-to-obtain reagents, simple operation, and rapid response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A preparation process for rivaroxaban, comprising the steps of:
[0028] S1. Add phthalimide potassium salt (73.4g, 0.39mol) to a solution of (S)-4-chloro-3-hydroxybutyronitrile (39.5g, 0.33mol) in DMF (330ml), heat After reacting at 70°C for 4h, pour the reaction solution into water (440ml), stir for 10min, a white solid precipitated out, filter with suction, and dry the filter cake under reduced pressure to obtain a white solid (73.6g, 97.0%); mp137~140°C , [α]D 25 -21.8°(c1, CHCl 3 ). ESI-MS(m / z):231[M+H] + ; 1 H NMR (300MHz, DMSO-d 6 )δ: 7.88(d, J=8.9Hz, 2H), 7.85(d, J=8.9Hz, 2H), 4.23~4.26(m, 1H), 3.63(d, J=5.6Hz, 2H), 3.34( s,1H), 2.62~2.68(m,2H), compared with the literature, it can be known as (S)-4-(1,3-dioxoisoindol-2-yl)-3-hydroxybutyronitrile;
[0029] S2. Add (S)-4-(1,3-dioxoisoindol-2-yl)-3-hydroxybutyronitrile (63.4g, 0.28mol) obtained in step S1 to chloroform (330ml), Under the condition of ice-water bath, add 30% hydrogen peroxide (122ml, 3.95mo...
Embodiment 2
[0035] A preparation process for rivaroxaban, comprising the steps of:
[0036] S1. Add phthalimide potassium salt (60.0g, 0.32mol) to a solution of (S)-4-chloro-3-hydroxybutyronitrile (32.3g, 0.27mol) in DMF (270ml), heat React at 70°C for 4h, pour the reaction solution into water (360ml), stir for 10min, a white solid precipitates out, filter with suction, and dry the filter cake under reduced pressure to obtain a white solid (60.2g, 96.6%); mp137~140°C , [α]D 25 -21.8°(c1, CHCl 3 ). ESI-MS(m / z):231[M+H] + ; 1 H NMR (300MHz, DMSO-d 6 )δ: 7.88(d, J=8.9Hz, 2H), 7.85(d, J=8.9Hz, 2H), 4.23~4.26(m, 1H), 3.63(d, J=5.6Hz, 2H), 3.34( s,1H), 2.62~2.68(m,2H), compared with the literature, it can be known as (S)-4-(1,3-dioxoisoindol-2-yl)-3-hydroxybutyronitrile;
[0037] S2. Add (S)-4-(1,3-dioxoisoindol-2-yl)-3-hydroxybutyronitrile (51.8g, 0.22mol) obtained in step S1 to chloroform (270ml), Under the condition of ice-water bath, add 30% hydrogen peroxide (100ml, 2.96mol), tetra...
Embodiment 3
[0043] A preparation process for rivaroxaban, comprising the steps of:
[0044] S1. Add phthalimide potassium salt (66.7g, 0.36mol) to (S)-4-chloro-3-hydroxybutyronitrile (35.9g, 0.3mol) in DMF (300ml) solution, heat After reacting at 70°C for 4h, pour the reaction solution into water (400ml), stir for 10min, a white solid precipitated out, filter with suction, and dry the filter cake under reduced pressure to obtain a white solid (66.9g, 96.8%); mp137~140°C , [α]D 25 -21.8°(c1, CHCl 3 ). ESI-MS(m / z):231[M+H] + ; 1 H NMR (300MHz, DMSO-d 6 )δ: 7.88(d, J=8.9Hz, 2H), 7.85(d, J=8.9Hz, 2H), 4.23~4.26(m, 1H), 3.63(d, J=5.6Hz, 2H), 3.34( s,1H), 2.62~2.68(m,2H), compared with the literature, it can be known as (S)-4-(1,3-dioxoisoindol-2-yl)-3-hydroxybutyronitrile;
[0045] S2. Add (S)-4-(1,3-dioxoisoindol-2-yl)-3-hydroxybutyronitrile (57.6g, 0.25mol) obtained in step S1 to chloroform (300ml), Under the condition of ice-water bath, add 30% hydrogen peroxide (110ml, 3.59mol), te...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chiral purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com