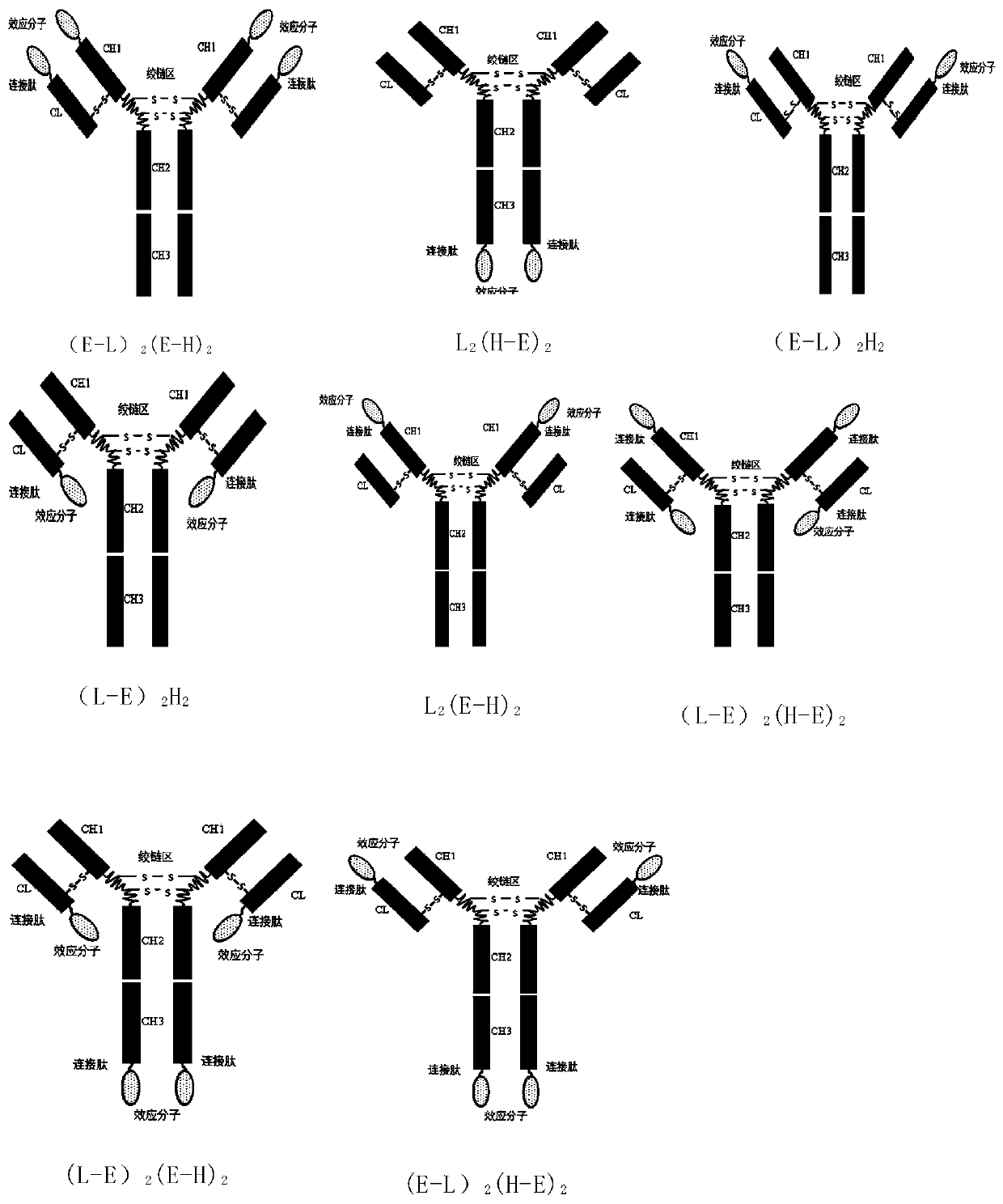
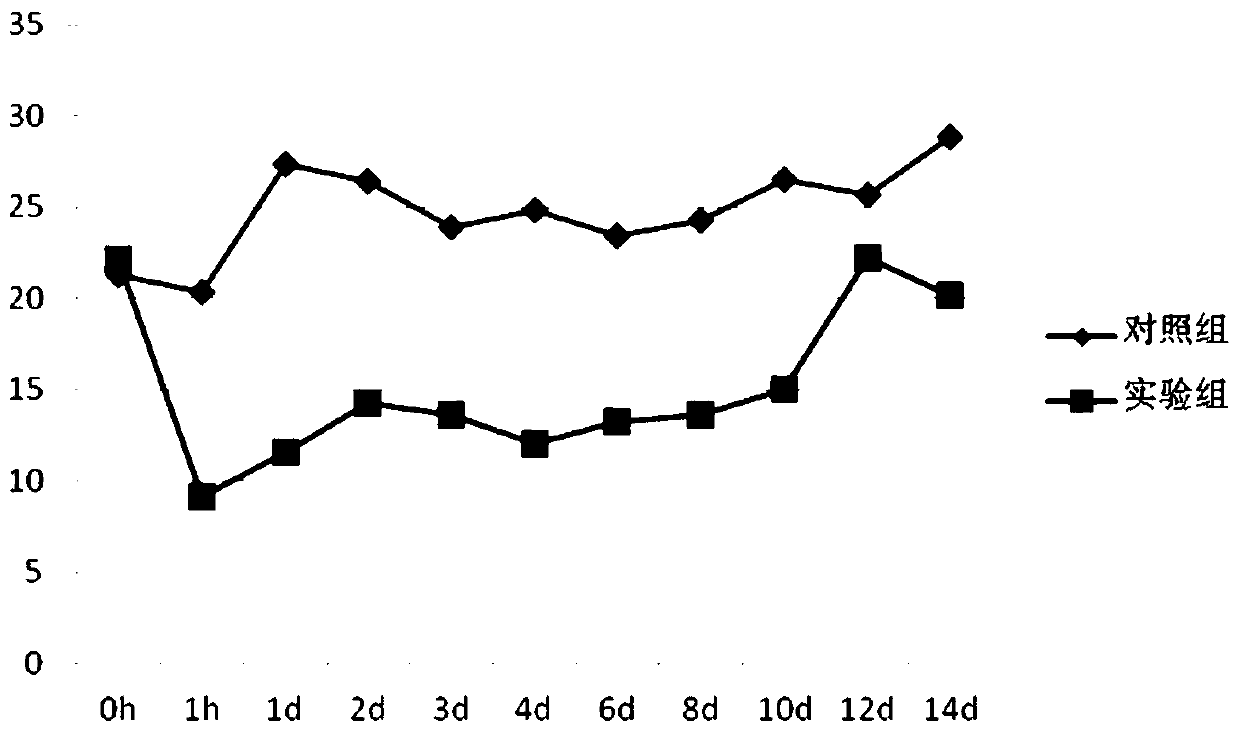
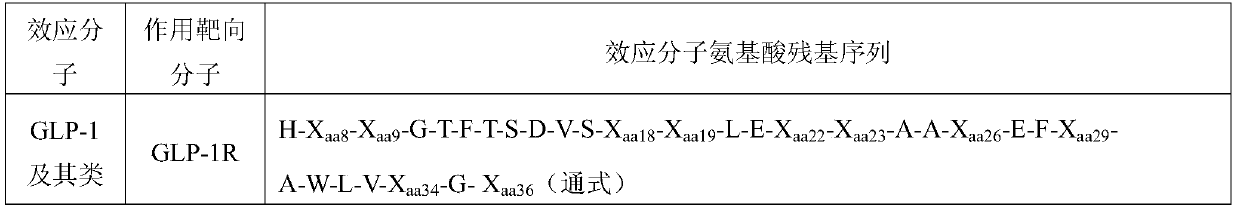
igg-like long-acting immune fusion protein and its application
A fusion protein and protein technology, applied in the biological field, can solve the problem of short biological half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1, the construction of high-efficiency expression vector of glutamine synthetase
[0057] 1. Cloning of gene encoding diabetes-associated IgG-like immune fusion protein and construction of expression vector
[0058] 1. Synthesis of dulaglutide-encoding gene and construction of expression vector
[0059] Dulaglutide (Trulicity) is an FDA-approved glucagon-like peptide-1 (GLP-1) receptor agonist and an immune fusion protein used to help normalize blood sugar levels , whose structure is GLP-1-Fc, which belongs to the existing typical Fc immune fusion protein.
[0060] 根据International Nonproprietary Name(INN)数据库中和专利中有关Dulaglutide的氨基酸残基序列“HGEGTFTSDVSSYLEEQAAKEFIAWLVKGGGGGGGSGGGGSGGGGSAESKYGPPCPPCPAPEAAGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSQEDPEVQFNWYVDGVEVHNAKTKPREEQFNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKGLPSSIEKTISKAKGQPREPQVYTLPPSQEEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSRLTVDKSRWQEGNVFSCSVMHEALHNHYTQKSLSLSLG(序列52)”委托上海捷瑞生物工程有限公司合成将Dulaglutide的编码基因“CACGGAGAGGGCAC...
Embodiment 2
[0092] Example 2, Screening, expression and purification of IgG-like immune fusion protein expressing cells
[0093] 1. Screening of cells expressing IgG-like immune fusion proteins
[0094] 1. CHO-K1 cells (ATCC, catalog number: CCL-61) were adapted and grown adherently in CD OptiCHO (Invitrogen product, catalog number: 12681) with 10% D-FBS and 4 mM glutamine.
[0095] 2. After 24 hours, according to the instructions of Lipofectin 2000 (Invitrogen product number: 11668-019), transfect the cells with the recombinant vector constructed in Example 1, and carry out in a 10 cm culture dish: wash with serum-free and antibiotic-free IMDM medium for 2 Each time, IMDM 100 μl / well was added each time. The expression plasmid constructed in Example 1 was mixed evenly with the IMDM medium, and the Lipofectin 2000 was mixed evenly with the IMDM medium. After standing for 5 minutes, mix the two. After standing at room temperature for 15 minutes, the Lipofectin 2000CD and expression plas...
Embodiment 3
[0101] Example 3, Biacore 3000 detects the affinity of IgG-like immune fusion protein and its target
[0102] 1. Experimental method
[0103] 1. Fixed
[0104] Dilute GLP-1R, VEGF-A, VEGF-B and TNF-α (all Yiqiao Shenzhou products) to a concentration of 10 μg / ml, and use amino coupling method to covalently immobilize on carboxymethyl-dextrin via primary amine Sugar-coated CM5 chip (General Electric product), fixation buffer 10mM sodium acetate (pH5.0), fixation amount is 1000RU.
[0105] 2. Regeneration conditions
[0106] Regeneration solution: glycine buffer solution with a pH of 1.5-2.0; injection time: 30s; flow rate: 30 μl / min.
[0107] 3. Kinetic analysis
[0108] PBST (recipe: Tween 20 containing 0.005% by volume in PBS) is the Running buffer; use the Kinetic Analysis Wizard mode; dilute Dulaglutide, BY23.2, BY25 and BY19.3 to 600, 300, 150, Concentration gradient of 75, 37.5 and 18.75nM; injection time: 2min, dissociation time: 5min, flow rate: 30μl / min.
[0109] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com