Preparation method of efficient and low-toxicity acaricide hexythiazox
A high-efficiency, low-toxic, and hexythifen technology, applied in the field of acaricides, can solve the problems of being unsuitable for industrial production, long reaction steps, cumbersome operations, etc., and achieve the effects of improving production environment, mild reaction, and reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
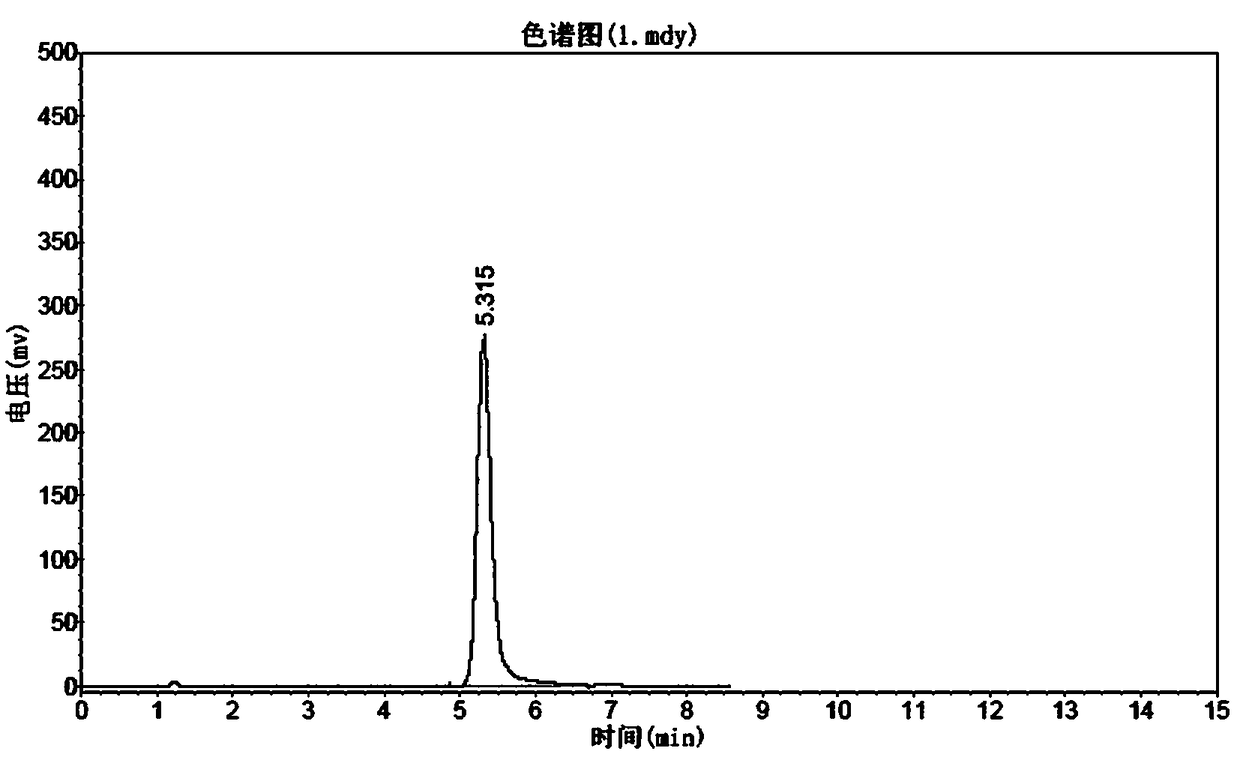
Image
Examples
Embodiment 1
[0041] The preparation method of a high-efficiency and low-toxicity acaricide hexythiazox according to the present invention includes the following steps:
[0042] (1) Oximation reaction
[0043] Put 200ml of methanol, 34.06g (0.2mol) of p-chloropropiophenone, 14.46g (0.204mol) of hydroxylamine hydrochloride into a 500ml four-necked flask, raise the temperature for reflux reaction, after the reaction, cool down, add 8.08g (0.2mol) of sodium hydroxide Sum up to Ph=7, then recover methanol under reduced pressure, add 200ml of toluene and 100ml of water, stir to dissolve for 30min, stand to separate the layers, separate the water layer, the organic phase is heated and refluxed to remove the water to obtain p-chlorophenylpropoxime toluene liquid;
[0044] (2) Rearrangement reaction
[0045] Add 20.44g (0.2mol) of triethylamine to the toluene solution of p-chlorophenylpropoxime obtained from the previous oximation reaction, reduce the temperature to 5±3℃, and add 23.38g (0.2mol) of methan...
Embodiment 2
[0057] The preparation method of a high-efficiency and low-toxicity acaricide hexythiazox according to the present invention includes the following steps:
[0058] (1) Oximation reaction
[0059] Put 200ml of methanol, 34.06g (0.2mol) of p-chloropropiophenone (0.2mol), and 17.02g (0.24mol) of hydroxylamine hydrochloride into a 500ml four-necked flask. The reaction is heated and refluxed. After the reaction is completed, the temperature is lowered. Add potassium hydroxide 14.93g (0.24mol) Sum to Ph=7, then recover methanol under reduced pressure, add 200ml of toluene and 100ml of water, stir to dissolve for 30min, stand to separate the layers, separate the water layer, heat the organic phase to reflux and remove the water to obtain p-chlorophenylpropoxime toluene liquid;
[0060] (2) Rearrangement reaction
[0061] Add 22.49g (0.22mol) of triethylamine to the toluene solution of p-chlorophenylpropoxime obtained from the previous oximation reaction, reduce the temperature to 5±3℃, and ...
Embodiment 3
[0073] The preparation method of a high-efficiency and low-toxicity acaricide hethiazolone according to the present invention includes the following steps:
[0074] (1) Oximation reaction
[0075] Put 200ml of methanol, 34.06g (0.2mol) of p-chloropropiophenone, 17.02g (0.24mol) of hydroxylamine hydrochloride into a 500ml four-necked flask, raise the temperature for reflux reaction, after the reaction, cool down, add sodium hydroxide 9.79g (0.24mol) Sum to Ph=7, then recover methanol under reduced pressure, add 200ml of toluene and 100ml of water, stir to dissolve for 30min, stand to separate the layers, separate the water layer, heat the organic phase to reflux and remove the water to obtain p-chlorophenylpropoxime toluene liquid;
[0076] (2) Rearrangement reaction
[0077] Add 21.46g (0.21mol) of triethylamine to the toluene solution of p-chlorophenylpropoxime obtained from the previous oximation reaction, control the temperature to 15±3℃, and add 25.45g (0.22mol) of methanesulfony...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com