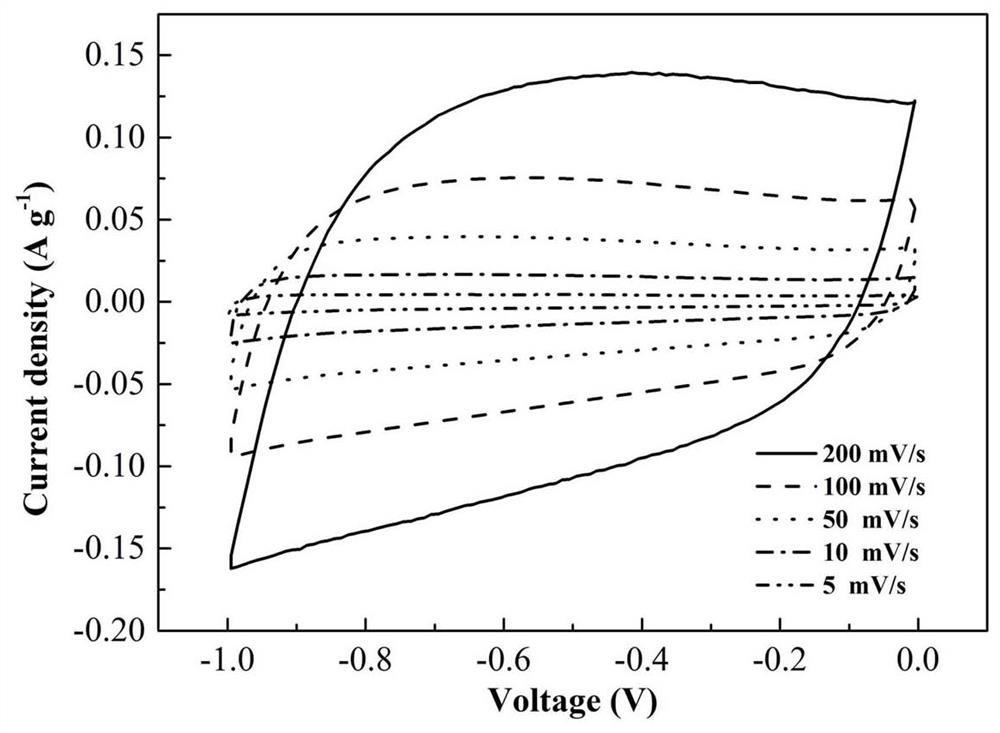
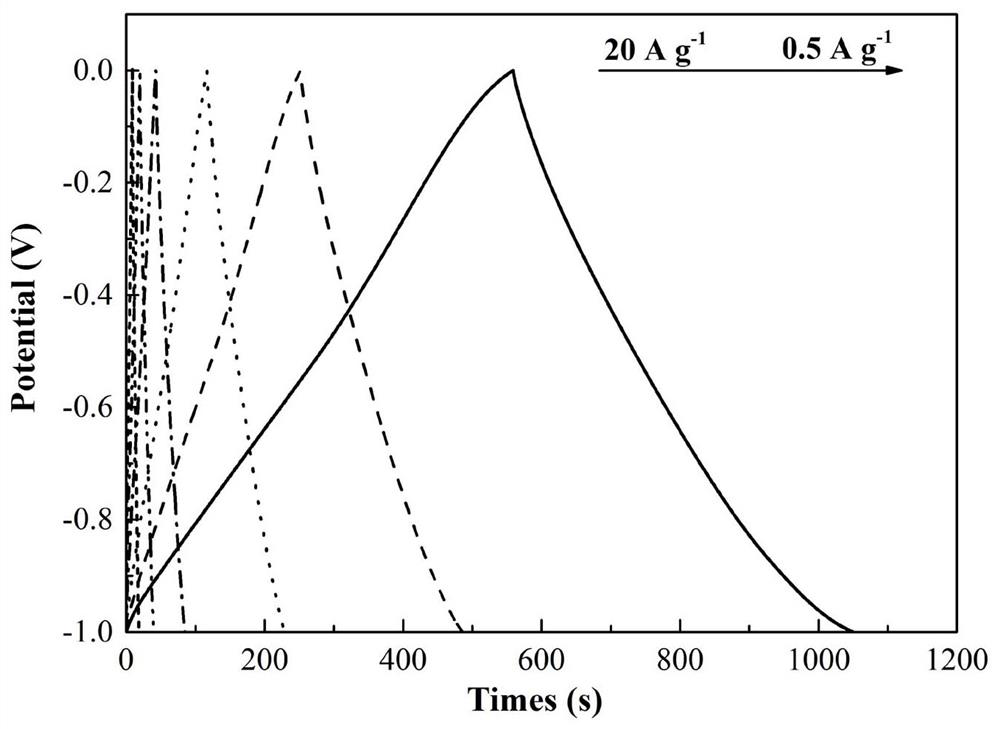
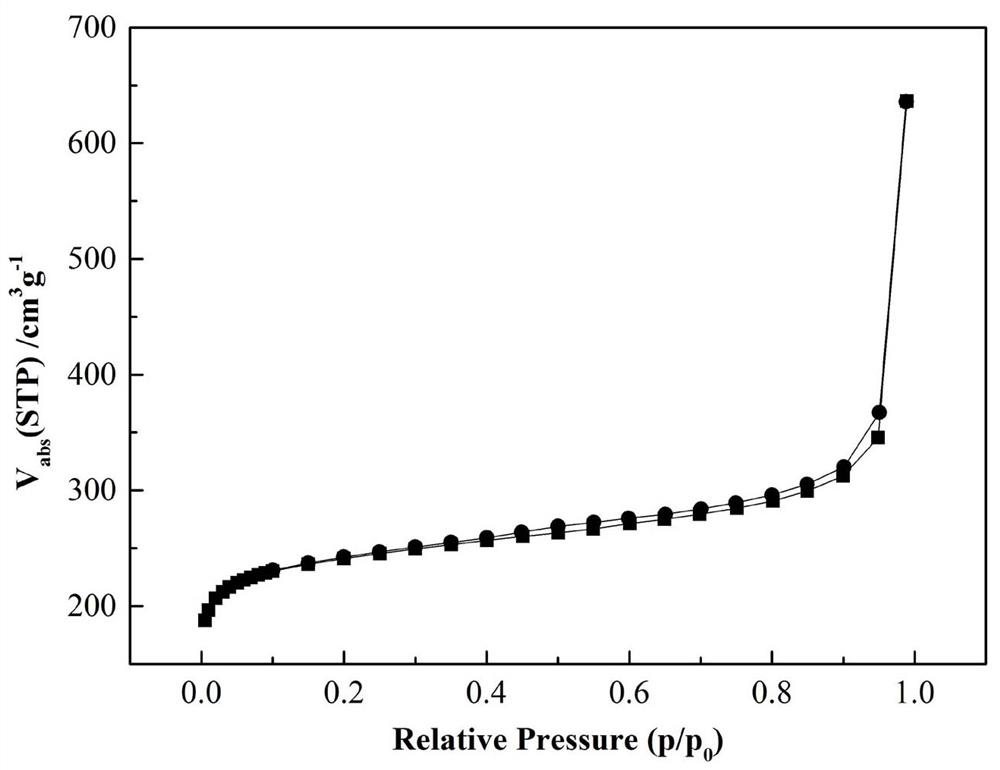
A nitrogen-boron co-doped porous carbon material based on breaking the bn bond and its preparation method and application
A porous carbon material and co-doping technology, which is applied in the preparation/purification of carbon, can solve the problems of long time consumption and complicated preparation process, and achieve the effects of high specific capacitance, simple synthesis process and simplified doping process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0037] A method for preparing a nitrogen-boron co-doped porous carbon material based on breaking the BN bond:
[0038] Step 1) Mix sodium hydroxide, resorcinol, formaldehyde, and boron nitride in a mass ratio of 0.02:1:2:3, weigh 0.02g of sodium hydroxide, 2.2g of resorcinol, 4.4g of formaldehyde, and nitrogen 3 g of boron chloride was prepared into a solution, stirred at room temperature for 4 hours, and hydrothermally reacted at 160°C for 24 hours to obtain a nitrogen-containing boron gel;
[0039] Step 2) Freeze-dry the nitrogen-boron-containing gel at minus 40 degrees for 48 hours to obtain a dried nitrogen-boron-containing gel;
[0040] Step 3) Mix the dry nitrogen-containing gel with the basic inorganic substance potassium hydroxide at a mass ratio of 1:2, add an appropriate amount of distilled water, and grind at room temperature for 0.5 hours to obtain activated nitrogen-doped boron porous carbon;
[0041] Step 4) Carbonize the nitrogen-doped boron precursor in a nitr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com