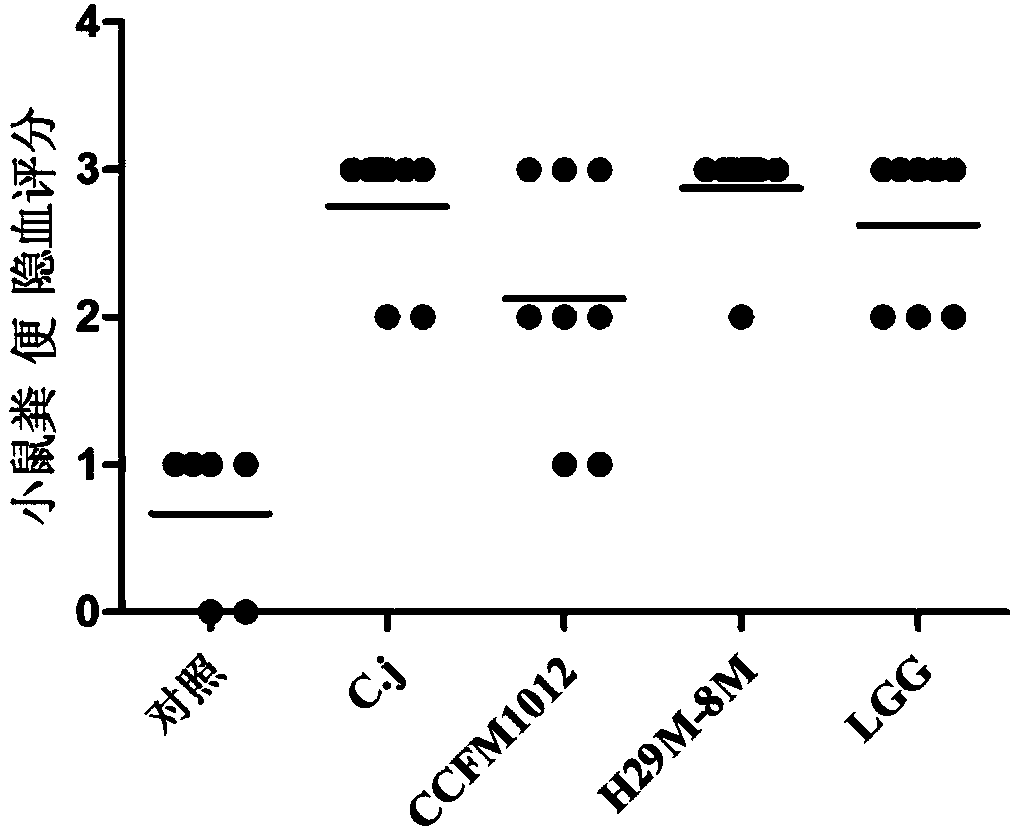
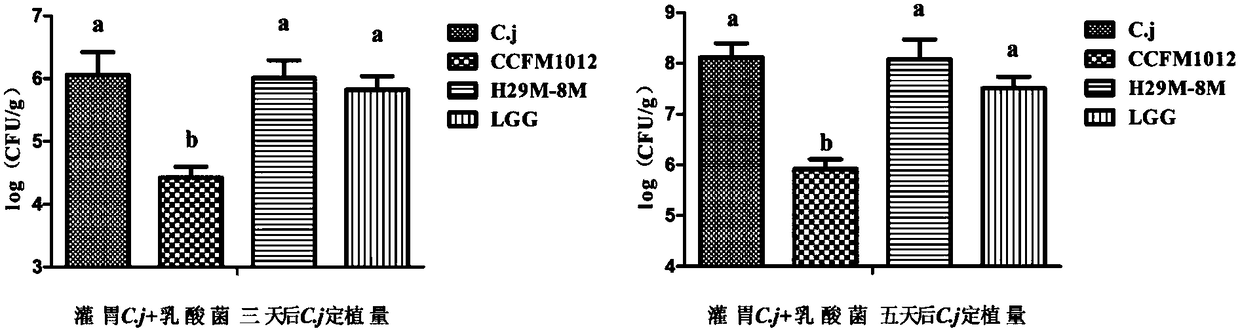
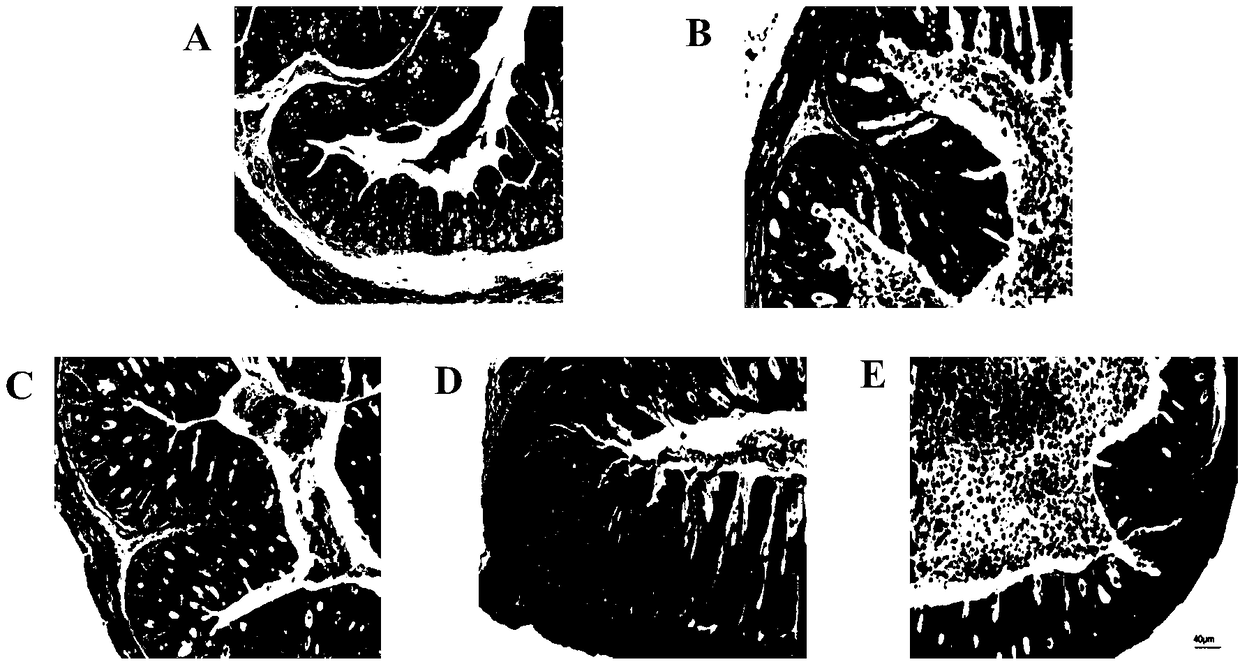
Pediococcus pentosaceus CCFM1012 as well as fermented food thereof and application thereof in preparation of medicine for antagonizing campylobacter jejuni infection
A CCFM1012, Campylobacter jejuni technology, applied in the field of microorganisms, can solve problems such as antibiotic resistance and antibiotic residues, and achieve the effects of good gastric acid resistance, prolong life, and good adhesion ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1: Tolerance of Pediococcus pentosaceae CCFM1012 to simulated gastrointestinal fluid
[0069] The cryopreserved Pediococcus pentosaceae CCFM1012 was streak-inoculated in the MRS solid medium, cultured aerobically at 37°C for 48 hours, and then subcultured in the MRS culture medium for 2 to 3 times, then the culture medium of Pediococcus pentosaceae CCFM1012 was taken, The bacteria were collected by centrifugation at 8000r / min for 5min, resuspended in (1:1) artificial simulated gastric juice of pH 2.5 (MRS medium containing 1% pepsin, pH=2.5), and then cultured anaerobically at 37°C. Samples were taken at the beginning (0h), 1h, 2h and 3h, and MRS agar medium was used to pour culture for plate colony counting, and the number of viable bacteria was determined and the survival rate was calculated. The survival rate is the ratio of the number of viable bacteria in the culture medium to the number of viable bacteria at 0h, expressed in %.
[0070] Get the culture so...
Embodiment 2
[0076] Example 2: Pediococcus pentosaceae CCFM1012 inhibits the growth of Campylobacter jejuni in vitro
[0077] Take Pediococcus pentosaceae CCFM1012, Pediococcus pentosaceae H29M-8M and Lactobacillus rhamnosus LGG out of the refrigerator at -80°C, streak on the MRS plate, culture at 37°C for 48 hours, pick a single colony in the MRS liquid tube, cultured at 37°C for 18 hours, inoculated into new MRS liquid medium at a volume of 2%, cultivated at 37°C for 18 hours, and cultured for another generation in the same way, then the lactic acid bacteria suspension was mixed at 8000r / min, 4 Centrifuge at ℃ for 8 min, absorb the supernatant, filter and sterilize with a 0.22 μm water filter membrane to obtain the supernatant of lactic acid bacteria fermentation, and adjust the fermentation liquid to pH = 6.5 with 1 mol / L NaOH.
[0078] Campylobacter jejuni NCTC11168 strain (purchased from the American Type Culture Collection ATCC) was cultured on two media (namely, Brooke's agar (produ...
Embodiment 3
[0084] Example 3: Adhesion ability of Pediococcus pentosaceae CCFM1012 to intestinal epithelial cells HT-29
[0085] The intestinal epithelial cell line HT-29 cells (purchased from the Cell Bank of the Type Culture Collection Committee of the Chinese Academy of Sciences) were cultured using RPMI1640 medium (Gibco Company) (supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin). HT-29 cells in 5% CO 2 The culture was carried out in a cell incubator (37° C.), and the culture medium was changed once every 48 hours of culture, and the culture was continuous.
[0086] Digest the HT-29 cells grown to 70%-80% confluence and adjust the concentration to 2×10 5 cells / mL, place sterile coverslips in a 6-well cell culture plate, add 2 mL of cell culture suspension to each well, and store in 5% CO 2 Culture in an incubator at 37°C. When the cells grow to a monolayer, wash with PBS three times, and add 1mL containing 2×10 8 CFU / mL lactic acid bacteria RPMI-1640 cell cul...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com