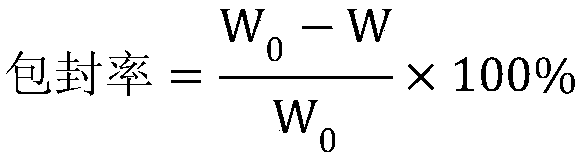Method for preparing liposome
A technology of liposomes and blank liposomes, which is applied in liposome delivery, sanitary equipment for toilets, medical preparations of non-active ingredients, etc., can solve problems such as loss, control content loss, and improve encapsulation rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1, the preparation of irinotecan hydrochloride liposome injection
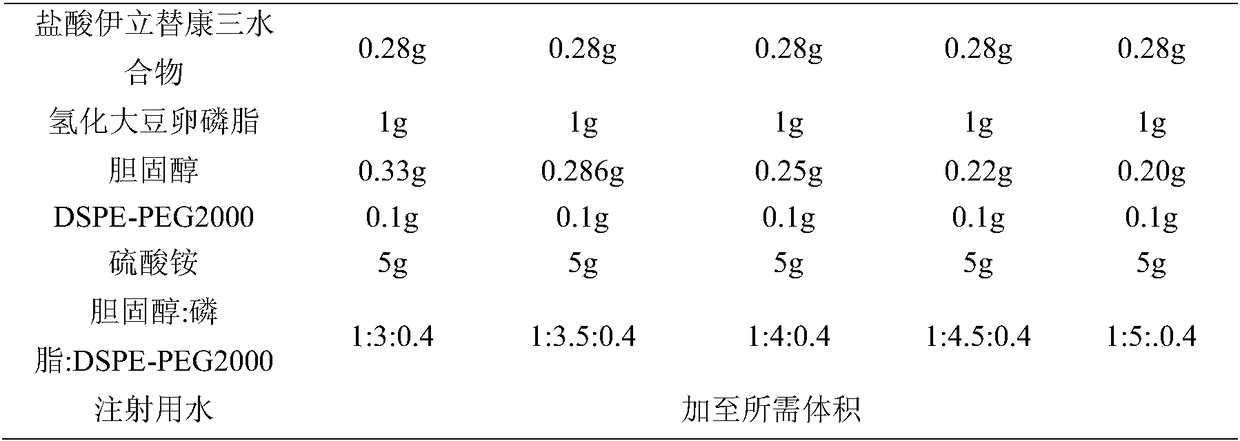
[0041] prescription
[0042] Table 1. Comparison of different irinotecan hydrochloride liposome prescriptions
[0043]
[0044] Preparation:
[0045] Dissolve the prescribed amount of hydrogenated soybean lecithin (HSPC) and cholesterol (CHOL) in an appropriate amount of absolute ethanol to obtain a lipid solution, mix it with 100mL of ammonium sulfate solution, remove the ethanol under reduced pressure to obtain a crude blank liposome; use high-pressure homogenization Machine 1000bar homogenization for 5 cycles, and then extrude liposomes to control its particle size through extrusion equipment (extruder spreads 2 pieces of 0.1 μm extrusion film, extrude 5 times); add the prepared DSPE-PEG2000 aqueous solution, Stir and incubate for 20 minutes, dialyze the blank liposomes with a tangential flow ultrafiltration device, and supplement phosphate buffer saline continuously in the middle to ...
Embodiment 2
[0047] Embodiment 2, the investigation of sterilization filter condition
[0048] (1) Determination conditions of drug sample content:
[0049] Chromatographic conditions: the chromatographic column is filled with octadecylsilane bonded silica gel (Ultimate XB-C18, 250×4.6mm, 5μm);
[0050] Mobile phase: 0.05mol / L phosphate buffer (take 6.8g of potassium dihydrogen phosphate, add 800mL of water, 10mL of triethylamine, dropwise add phosphoric acid to adjust the pH to 4.0, add water to 1000mL to obtain)-methanol-acetonitrile ( 45:55:5, volume ratio) is mobile phase;
[0051] The detection wavelength is 254nm, the flow rate is 1.0mL / min, and the column temperature is 30°C;
[0052] Injection volume: 20μL;
[0053] Record chromatogram, calculate the content of irinotecan hydrochloride trihydrate in need testing solution by external standard method.
[0054] (2) Determination conditions of edetate calcium and sodium content:
[0055] Chromatographic conditions: the chromatogra...
Embodiment 3
[0090] Embodiment 3, filter element drying treatment, the impact of residual moisture on drug content
[0091] The filter element needs to be sterilized before use. After sterilization, soak it in water for 30 minutes to check whether the bubble point is qualified, and then put it in a hot electric oven to dry. Install the dried filter to the corresponding position according to the mark, open the valves related to the compressed air heat exchange and the filter inlet, and purge each filter element for 70 minutes. After the end, take out the filter and weigh it. weight difference.
[0092] Content determination conditions:
[0093] Chromatographic conditions: the chromatographic column is filled with octadecylsilane bonded silica gel (Ultimate XB-C18, 250×4.6mm, 5μm);
[0094] Mobile phase: 0.05mol / L phosphate buffer (take 6.8g of potassium dihydrogen phosphate, add 800mL of water, 10mL of triethylamine, dropwise add phosphoric acid to adjust the pH to 4.0, add water to 1000m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com