Preparation method of Dapagliflozin isomer impurities I
A technology for isomers and impurities, applied in the field of organic synthesis, to achieve the effects of cost reduction, good reproducibility and less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1 compound A
[0033] Add 2-chloro-5-bromobenzoic acid (compound 2) (9.40g, 40mmol, 1.0eq) and dichloromethane (70mL) to a 250mL single-necked bottle, then add thionyl chloride (8.70mL, 120mmol, 3.0eq ) was stirred and reacted at 40° C. for 3 hours. After the reaction was completed, it was concentrated to dryness to obtain 10.20 g of compound A with a yield of 100%, which was directly used in the next reaction.
Embodiment 2
[0034] The preparation of embodiment 2 compound B
[0035] Add methoxymethylamine hydrochloride (9.75g, 100mmol, 2.5eq) and dichloromethane (90mL) into a 250mL single-necked bottle, stir and control the reaction temperature at -10-10°C, slowly add triethylamine ( 22mL, 160mmol, 4.0eq), after stirring for 30 minutes, slowly dropwise added the solution of the above compound A (10.20g, 40mmol, 1.0eq) dissolved in dichloromethane (15mL), after the dropwise addition was completed, continue to stir for 3 hours, recover At room temperature, the reaction solution was washed with 100 mL of water, separated, the organic phase was washed with 100 saturated sodium chloride, separated, the organic phase was dried over anhydrous Na2SO4, the solvent was removed under reduced pressure, and 11.0 g of white solid was obtained by vacuum drying, with a yield of 99% %, namely compound B.
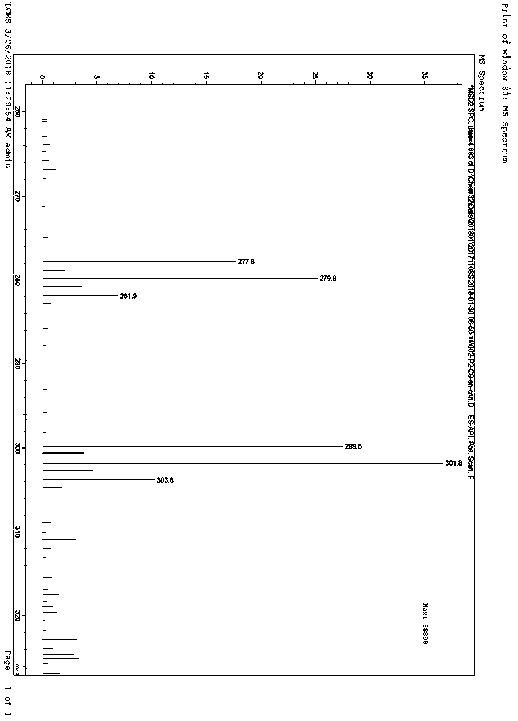
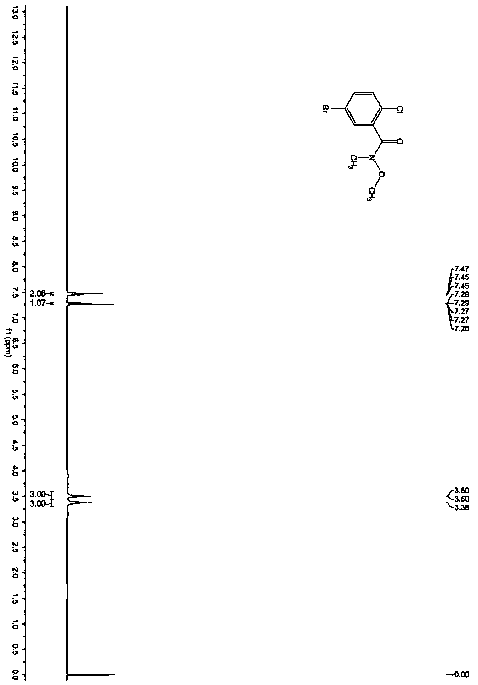
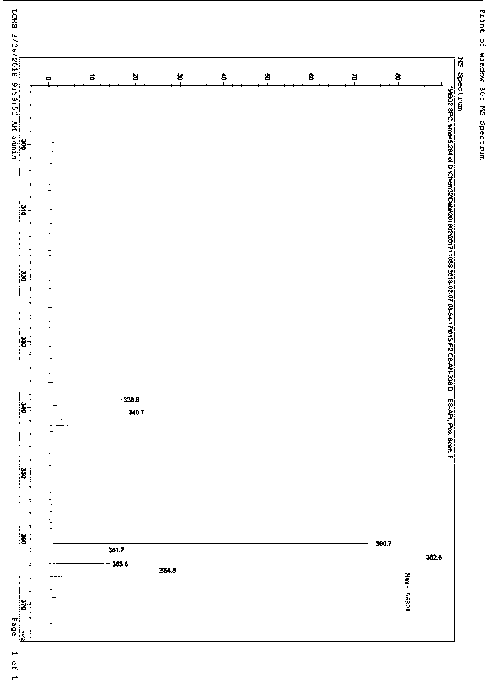
[0036] The product has passed the identification of MS spectrum and 1HNMR spectrum: as figure 1 and figur...
Embodiment 3
[0039] Embodiment 3 Preparation of Dapagliflozin Isomer Impurity I
[0040] Under nitrogen protection, add compound C (2.01g, 10mmol, 1.eq) in anhydrous tetrahydrofuran (20mL) solution to a 100mL three-necked flask, stir and control the reaction temperature at -78°C, slowly add 1.6M n-butyl Lithium hexane solution (6.9mL, 11mmol, 1.1eq), after the dropwise addition, was stirred for 30 minutes, and compound B (3.06g, 11mmol, 1.1 eq) solution, after the dropwise addition, stir to return to room temperature, react for 2 hours, add 50mL ice water and 50mL n-hexane to the reaction solution, separate the liquids, extract the aqueous phase with 30mL n-hexane, combine the organic phases, dry over anhydrous Na2SO4, and decompress The solvent was removed, and the residue was separated by column chromatography (mobile phase: PE:DCM=3:1~1:1) to obtain 3.13g of a white solid with a purity of 99.6% and a yield of 92%, which was the isomer of dapagliflozin Impurity I.
[0041] MS (m / z): 33...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com