A kind of preparation method of 5,6-dihydropyridin-2(1h)-one derivative
A technology of dihydropyridine and derivatives, applied in the field of medicinal chemistry, can solve the problems of low total yield, cumbersome operation, and large amount of phosphorus-containing wastewater, and achieve high product purity and yield, simple process operation, and cheap and easy raw materials The effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
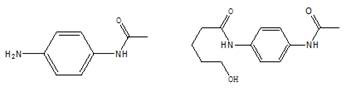
[0098] Example 1: Preparation of N-(5-hydroxyl-n-pentanoyl)-N'-acetyl p-phenylenediamine (Ⅲ)
[0099] In a 1000 ml four-neck flask connected with a stirrer, a thermometer, and a condenser tube, add 350 grams of toluene, 30 grams of N,N-dimethylformamide, 75.0 grams (0.5 moles) of para-acetamidoaniline (II), 70.0 Gram (0.7 moles) δ-valerolactone, heating, 110-115 ℃ of stirring reaction 4 hours, cooling to 20-25 ℃, filtering, 30 gram of toluene washing filter cakes, drying, obtains 115.8 gram of N-(5-hydroxyl N-pentanoyl)-N'-acetyl-p-phenylenediamine (Ⅲ), the yield is 92.6%, and the liquid phase purity is 99.5%.
Embodiment 2
[0100] Example 2: Preparation of N-(5-hydroxy-n-pentanoyl)-N'-acetyl p-phenylenediamine (Ⅲ)
[0101] To a 500 ml four-necked flask connected with a stirrer, a thermometer, and a condenser tube, add 250 g of N,N-dimethylformamide, 75.0 g (0.5 mole) of p-acetamidoaniline (II), 70.0 g (0.7 mole ) δ-valerolactone, heated, stirred at 115-120°C for 4 hours, cooled to 50-70°C, recovered N,N-dimethylformamide by distillation under reduced pressure, and recrystallized the residue with 400 grams of 75% methanol , filter, 30 grams of water wash the filter cake, dry to obtain 118.6 grams of N-(5-hydroxyl n-valeryl)-N'-acetyl p-phenylenediamine (Ⅲ), the yield is 94.9%, and the liquid phase purity is 99.9% %.
Embodiment 3
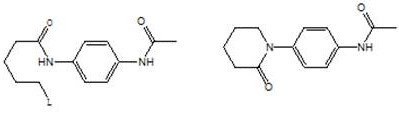
[0102] Example 3: Preparation of 4-(piperidin-2-one-1-yl)-N-acetylaniline (Ⅴ)
[0103] In the 500 milliliter four-neck flask that is connected with agitator, thermometer, reflux condenser and connected with 30wt% sodium hydroxide aqueous solution absorption device, add 150 grams of 1,2-ethylene dichloride, 25.0 grams (0.1 moles) implement N-(5-hydroxyl n-pentanoyl)-N'-acetyl p-phenylenediamine (Ⅲ) obtained by the method of example 2 is heated, and kept between 40-50°C of internal temperature, to which 23.8 grams (0.2 mol) of thionyl chloride and 50 g of 1,2-dichloroethane, the solution was added dropwise in 2 hours, and thereafter, the reaction was stirred at 55-60° C. for 3 hours. Cool to 30°C, change to a vacuum distillation device, and recover 1,2-dichloroethane and excess thionyl chloride by vacuum distillation (for the next batch reaction after analyzing the content), after the distillation is completed, cool to 20- At 25°C, the resulting residue N-(5-chloro-n-valeryl)-N...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chromatographic purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com