Fused DNA (Deoxyribonucleic Acid) and vaccine prepared from fused DNA
A DNA vaccine and vaccine technology, applied in the field of biomedicine, can solve the problems of inability to prevent latent infection and treatment of tuberculosis, complex preparation technology of subunit protein vaccines, and inability to be used in immunocompromised people, etc., to shorten the treatment period and reduce the risk of vaccination , easy to produce effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
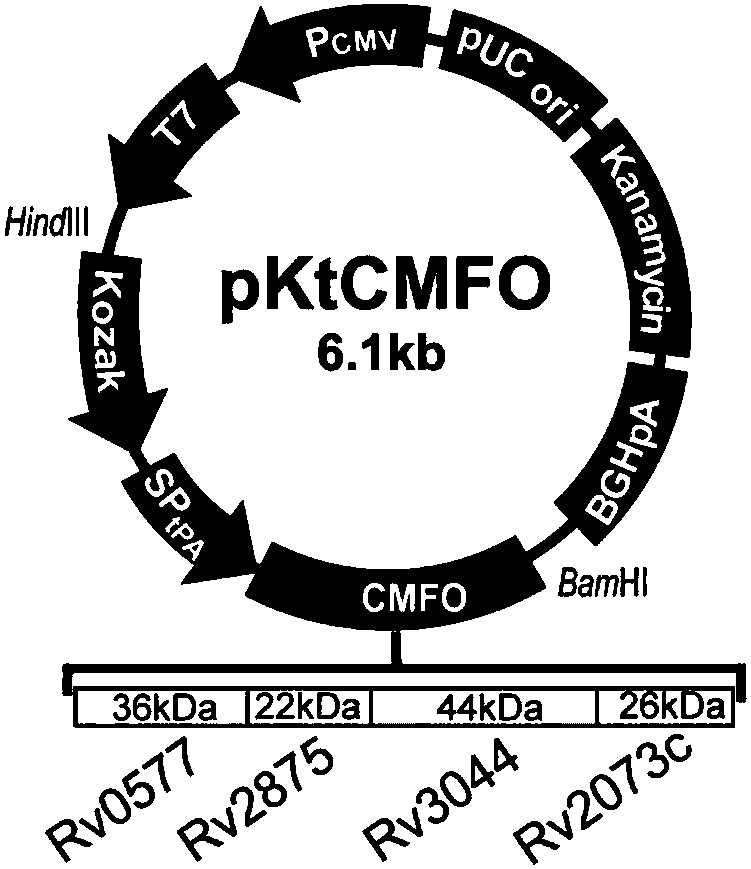
[0032] Embodiment 1 Design and synthesis of fusion gene CMFO and single antigen gene sequence and primer
[0033] The coding sequences of Rv0577, Rv2875, Rv3044 and Rv2073c of M.tb H37Rv were searched through Genbank. Use Signalp software to predict and search the UniProtKB database to see if there is a signal peptide in the search sequence, and then use DNAMAN software to analyze the restriction sites. A Kozak sequence (GCCACC) was added before the target gene to enhance the translation efficiency of the target gene. In order to secrete the final expression product outside the cell, a tPA signal peptide sequence is added before the spliced gene sequence. The splicing of the four target genes after the tPA signal peptide should follow the following principles: 1) If there is a signal peptide, the signal peptide should be removed, including the original start codon, and the stop codon should be removed at the same time (the last gene sequence after splicing Then add TAA); 2...
Embodiment 2
[0034] Embodiment 2 Construction of recombinant plasmid pVAX1-Koza-tPA-CMFO (pKtCMFO)
[0035] 1. Acquisition of the target gene:
[0036] (1) Primer design: Design primers based on the full sequence of the fusion gene and the multiple cloning site on the eukaryotic expression vector pVAX1. Store at ℃ for later use. See SEQ ID No.2 and SEQ ID No.3 for primer sequences.
[0037] (2) PCR reaction system:
[0038]
[0039]
[0040] (3) PCR reaction conditions:
[0041] 98℃30s; 98℃10s, 68℃20s and 72℃90s, 30cycles; 72℃10min; 4℃forever
[0042] 2. Construction of recombinant plasmids:
[0043] The PCR product recovered by DNA gel electrophoresis and the vector pVAX1 were subjected to double enzyme digestion respectively. The enzyme digestion system is:
[0044]
[0045] The KtCMFO fragment of the target gene recovered by enzyme digestion and the vector pVAX1 were ligated according to the following conditions:
[0046]
[0047] 3. Transformation and identification ...
Embodiment 3
[0049] Example 3 Recombinant plasmid pVAX1-CMFO (pCMFO), pVAX1-Koza-CMFO (pKCMFO), pVAX1-tPA-CMFO (ptCMFO), pVAX1-Koza-tPA-0577 (pKt0577), pVAX1-Koza-tPA-2875 (pKt2875 ), construction of pVAX1-Koza-tPA-3044 (pKt3044) and pVAX1-Koza-tPA-2073c (pKt2073c)
[0050] 1. Acquisition of the target gene:
[0051] (1) Primer design: Design primers based on the full sequence of the fusion gene and the multiple cloning site on the eukaryotic expression vector pVAX1. Store at ℃ for later use. See SEQ ID No.4-10 for the primer sequence.
[0052] (2) PCR reaction system:
[0053]
[0054] The template for amplifying the CMFO fragment is pDC316-KtCMFO, and the sequences of the upstream and downstream primers are P1: SEQ ID No.4; P2: SEQ ID No.3;
[0055] The template for amplifying the KCMFO fragment is pDC316-KtCMFO, and the upstream and downstream primer sequences are P1: SEQ ID No.5; P2: SEQ ID No.3;
[0056] The template for amplifying the tCMFO fragment is pDC316-KtCMFO, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com