Active polypeptide capable of promoting osteogenesis and inhibiting osteoclast and application of active polypeptide
A technology for inhibiting osteoclasts and active polypeptides, applied in the field of bone biomaterials and tissue engineering, can solve the problems of affecting selectivity and activity strength, not prominent conformation, and promoting fracture healing, so as to reduce osteoclasts and osteoporosis. , avoid risks and side effects, and facilitate the effect of large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
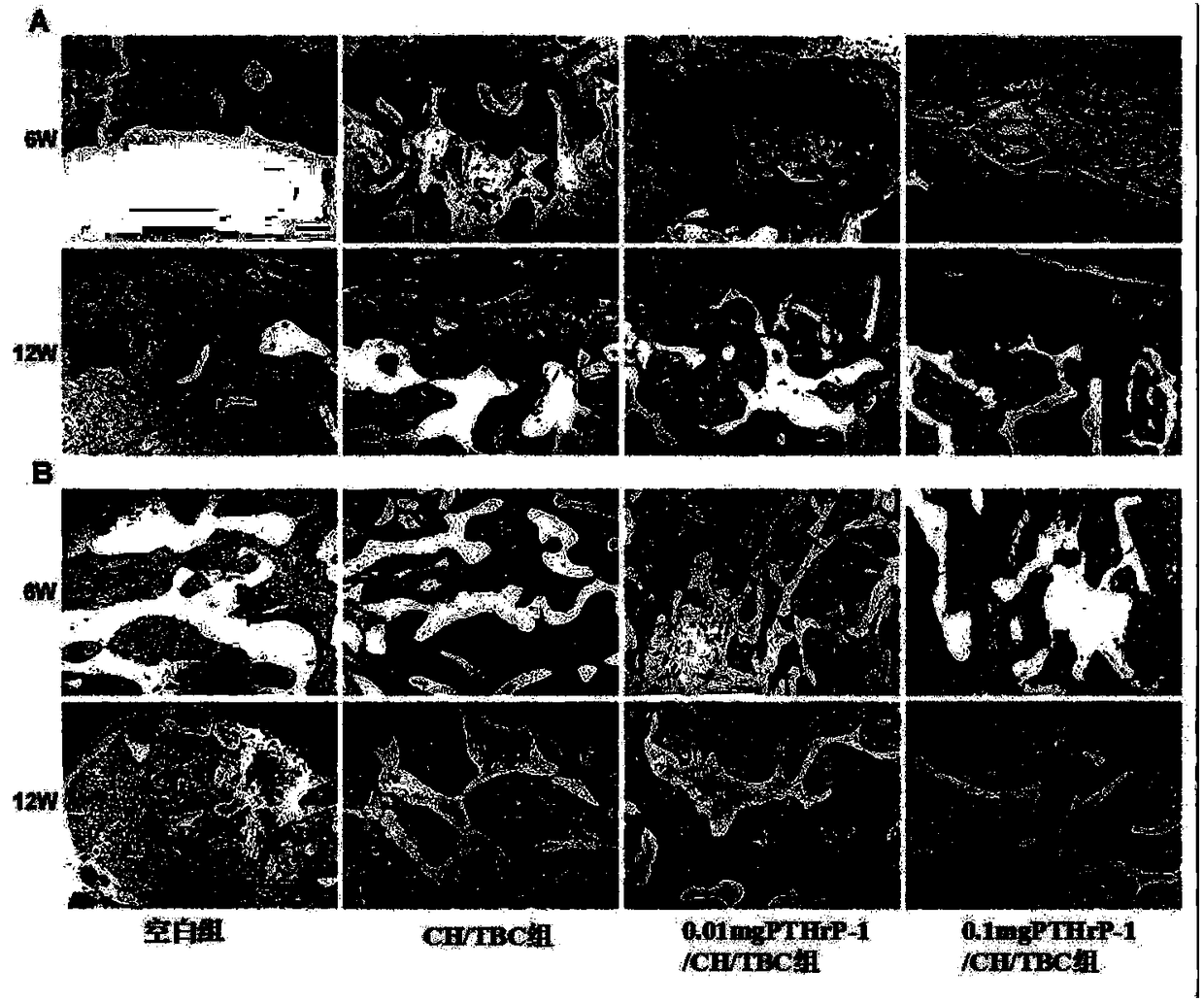
Image
Examples
Embodiment 1
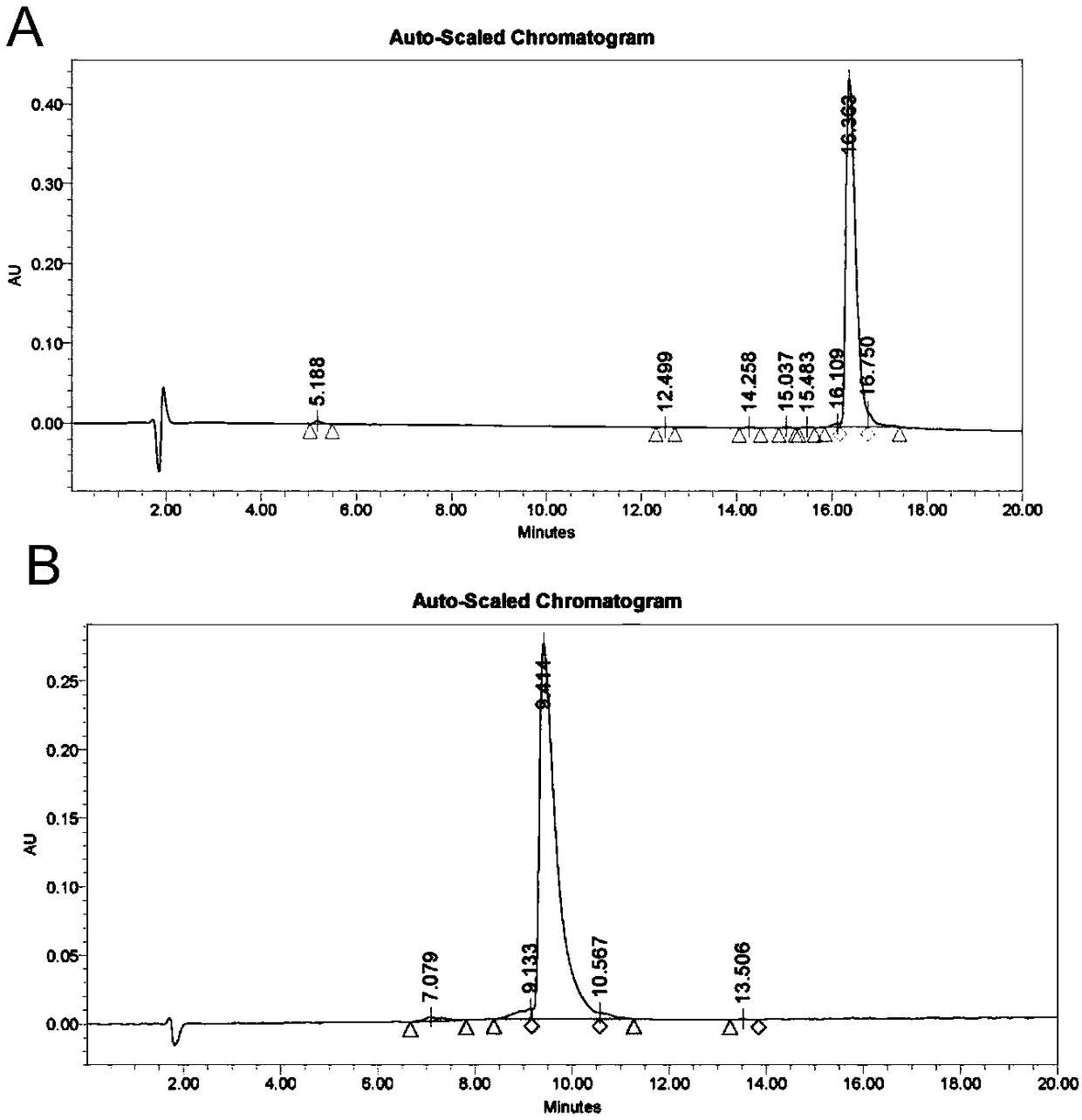
[0047] [Example 1] Synthesis of polypeptides PTHrP-1 and PTHrP-2
[0048] The polypeptide molecules required in the experiment were synthesized by an automatic peptide synthesizer, purified by high-performance liquid chromatography, and their purity and sequence were detected by mass spectrometry, amino acid and peptide analyzers. The sequences of PTHrP-1 and PTHrP-2 are respectively and The purity obtained by synthesis is greater than 95%, the molecular weight is 4543.07Da, and the molecular weight of PTHrP-2 is 4585.15Da. HPLC and MS such as figure 1 , figure 2 shown.
Embodiment 2
[0049] [Example 2] Toxicity test of polypeptide PTHrP-1
[0050] 1. Local skin irritation test
[0051] Take 3 healthy New Zealand white rabbits, male or female, weighing 2.0-2.5kg, cut off the rabbit hair on the back, observe for 2 days, no scars and irritation on the skin, inject 0.2ml of PTHrP-1 polypeptide solution with a concentration of 100ng / ml and normal saline respectively Subcutaneously on both sides of the spine, 10 points were taken on each side, and the injection site was observed at three time points of 1d, 3d, and 7d after injection. Observation at the three time points of 1d, 3d, and 7d showed no redness, swelling, or erythema at the injection site of the PTHrP-1 polypeptide solution and normal saline, suggesting that the PTHrP-1 polypeptide was not irritating to the animal body.
[0052] 2. Acute toxicity test
[0053] Choose 10 healthy mice, weighing 20-25g, half male and half male, and raise them in separate cages. Each mouse was intraperitoneally injecte...
Embodiment 3
[0056] [Example 3] Optimal concentration of polypeptide PTHrP-1 to promote osteogenic differentiation of MC3T3-E1 cells
[0057] Four kinds of PTHrP-1 polypeptide medium containing different concentrations (0ng / ml, 50ng / ml, 100ng / ml, 200ng / ml) were added to 24-well culture plate, each with 5 wells. Take the 3rd generation mouse MC3T3-E1 cells, the density is 1×10 5 / ml, add 0.5ml cell suspension to each well, at 37℃, the volume fraction is 5% CO 2 Cultured in an incubator, the medium was replaced every 2 days. After culturing for 14 days, ALP staining was performed using the ALP kit. It was found that in the culture wells of the three groups containing PTHrP-1 polypeptide, MC3T3-E1 cells aggregated and grew in layers, the cell shape gradually changed from round or round to polygonal or cubic, the secretion of extracellular matrix increased significantly, ALP Staining shows that reddish-brown granules or tan or coffee-colored granules appear in the cytoplasm of the cells, wh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com