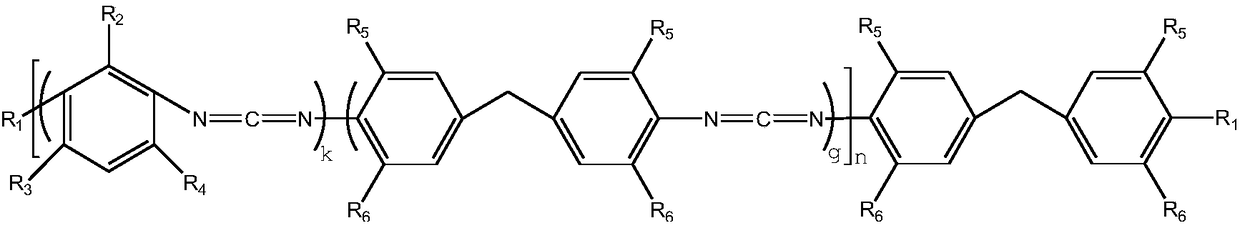
Random block type aromatic polycarbodiimide compound and preparation method thereof
A polycarbodiimide and random block technology, which is applied in the field of aromatic polycarbodiimide compounds and their preparation, can solve problems such as thermal instability, unfavorable addition, and easy volatility, and achieve improved hydrolysis resistance, The effect of improving mechanical properties and improving aging properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 200g of 2-methyl-4,6-diethylphenyl diisocyanate (0.22mol) and methylene bis(2-methyl-4-ethyl)phenylisocyanate (0.44 mol) was added to a 250ml three-necked flask and nitrogen gas was continuously introduced into it. After adding 100mg of catalyst 3-methyl-1-phenyl-2-phosphorus-1-oxide, the temperature of the system was heated to 140°C. Use the di-n-butylamine titration method to track the change of NCO in the system until the NCO content in the reaction system is 3.1%. Then, the catalyst in the system was removed by vacuum distillation at about 130°C. Finally, the temperature of the reaction system was lowered to 80° C. and 6.3 g of ethanol was added to react free NCO groups. The NCN content of the obtained polycarbodiimide is 13.6%, the degree of polymerization n is about 9, the melt color is light yellow and almost colorless transparent solid, and the appearance is white powder after crushing.
Embodiment 2
[0027]200g of 2-methyl-4,6-diethylphenyl diisocyanate (0.35mol) and methylene bis(2-methyl-4-ethyl)phenylisocyanate (0.35mol) in a molar ratio of 1:1 mol) was added to a 250ml three-necked flask and nitrogen gas was continuously introduced into it. After adding 100mg of catalyst 3-methyl-1-phenyl-2-phosphorus-1-oxide, the temperature of the system was heated to 140°C. Use the di-n-butylamine titration method to track the change of NCO in the system until the NCO content in the reaction system is 3.3%. Then, the catalyst in the system was removed by vacuum distillation at about 130°C. Finally, the temperature of the reaction system was lowered to 90° C. and 8.7 g of isopropanol was added to react free NCO groups. The NCN content of the obtained polycarbodiimide is 14.2%, the degree of polymerization n is about 9, the melt color is light yellow and almost colorless transparent solid, and the appearance is white powder after pulverization.
Embodiment 3
[0029] 200g of 2-methyl-4,6-diethylphenyl diisocyanate (0.50mol) and methylene bis(2-methyl-4-ethyl)phenylisocyanate (0.25 mol) was added to a 250ml three-necked flask and nitrogen gas was continuously introduced into it. After adding 100mg of catalyst 3-methyl-1-phenyl-2-phosphorus-1-oxide, the temperature of the system was heated to 140°C. Use the di-n-butylamine titration method to track the change of NCO in the system until the NCO content of the reaction system is 3.7%. Then, the catalyst in the system was removed by vacuum distillation at about 130°C. Finally, the temperature of the reaction system was lowered to 70° C. and 5.0 g of methanol was added to react free NCO groups. The NCN content of the obtained polycarbodiimide is 16.2%, the degree of polymerization n is about 9, the melt color is light yellow and almost colorless transparent solid, and the appearance is white powder after crushing.
PUM
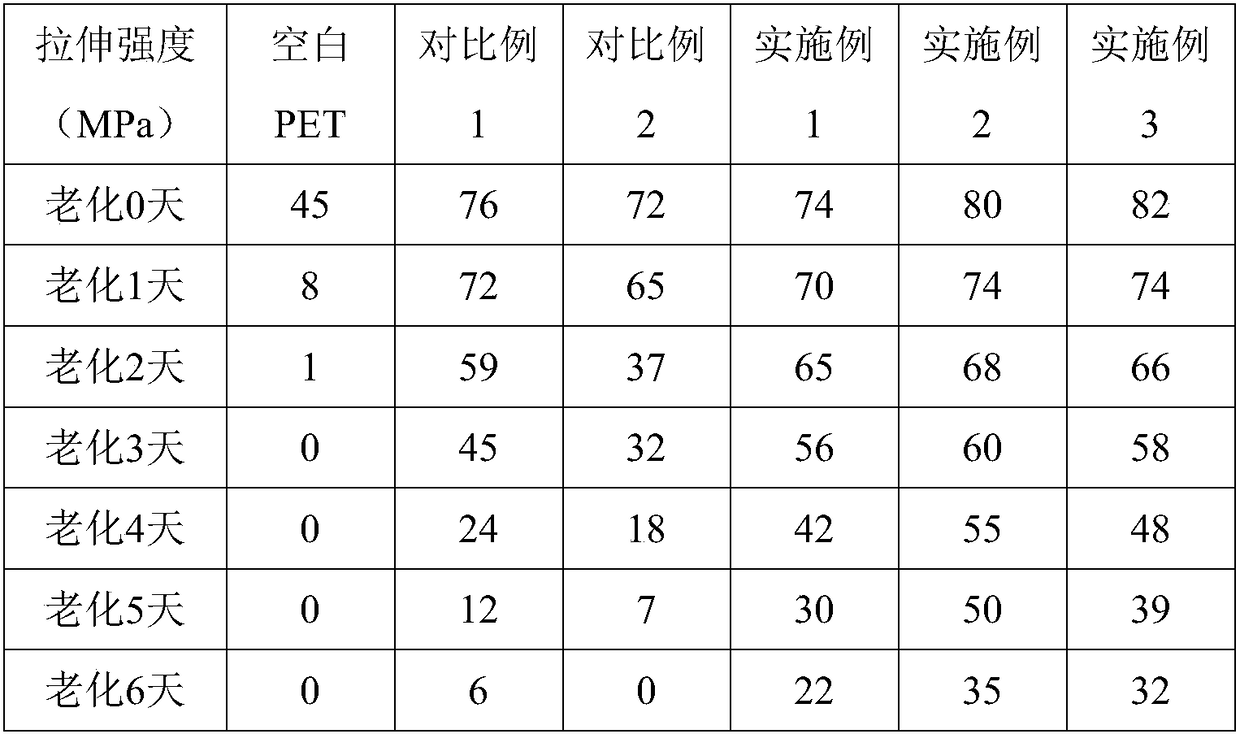
| Property | Measurement | Unit |
|---|---|---|
| Tensile strength | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com