Preparation method for beach-chair-typed nonacene compounds
A benzene compound and chair-type technology, which is applied in the field of preparation of beach chair-type nonacene compounds, can solve the problem of no nonacene, etc., achieve narrow energy band gap, high carrier mobility, and improve the ability to be easily oxidized and deteriorated. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The synthetic method of beach chair type nonacene adopts the following steps:
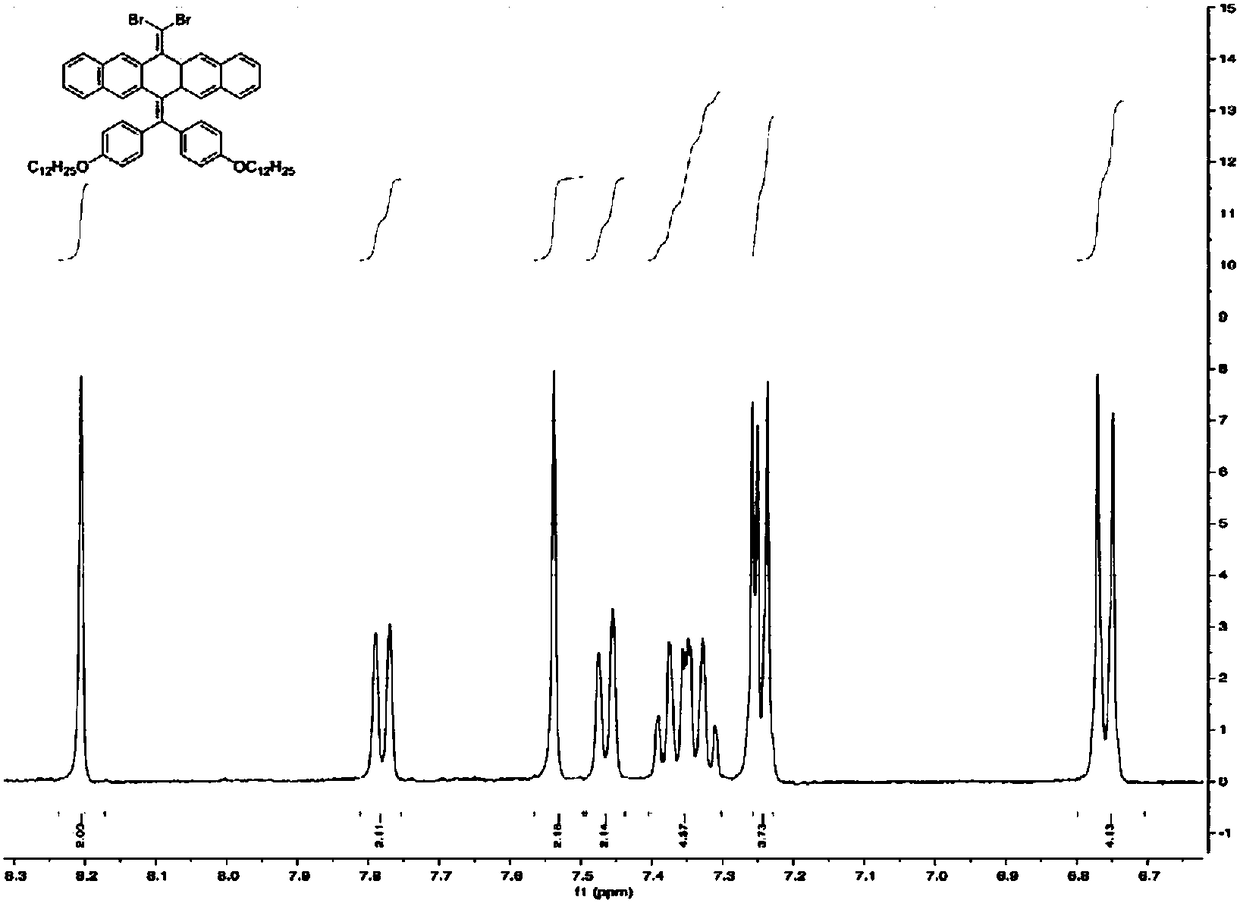
[0032] (1) Weigh CBr 4 (1.60g, 0.0048mol) was dissolved in 100mL toluene, stirred for 30min under nitrogen gas, and then PPh was added 3 (3.17g, 0.012mol), continue to keep N 2 Atmosphere, compound 1 (1.00 g, 0.0012 mol) was added after the color of the whole system turned light yellow. keep N 2 The atmosphere was heated to 80°C for 24h. After the reaction was completed, it was cooled to room temperature, and the reaction system was quickly suction-filtered, and the filtrate was spin-dried and then filtered through a fast silica gel column, and the filtrate was spin-dried to obtain compound 2.
[0033] (2) Weigh compound 2 (0.50g, 0.51mmol), phenylboronic acid (0.032g, 0.26mmol), potassium carbonate K 2 CO 3 (0.72g, 5.2mmol) in a sealed tube, add 50mL of toluene, 10mL of ethanol, 10mL of mixed solvent composed of water, nitrogen gas and stir for 30min, then add the catalyst Pd(PPh 3 )...
Embodiment 2
[0037] The synthetic method of beach chair type nonacene adopts the following steps:
[0038] (1) Weigh CBr 4 (2.40g, 0.0072mol) was dissolved in 100mL toluene, stirred for 20min under nitrogen gas, and then PPh was added 3 (4.76g, 0.018mol) continue to maintain N 2 Atmosphere, compound 1 (1.50 g, 0.0018 mol) was added after the color of the whole system turned light yellow. keep N 2 The atmosphere was heated to 110°C for 24h. After the reaction was completed, it was cooled to room temperature, and the reaction system was quickly suction-filtered, and the filtrate was spin-dried and then filtered through a fast silica gel column, and the filtrate was spin-dried to obtain compound 2.
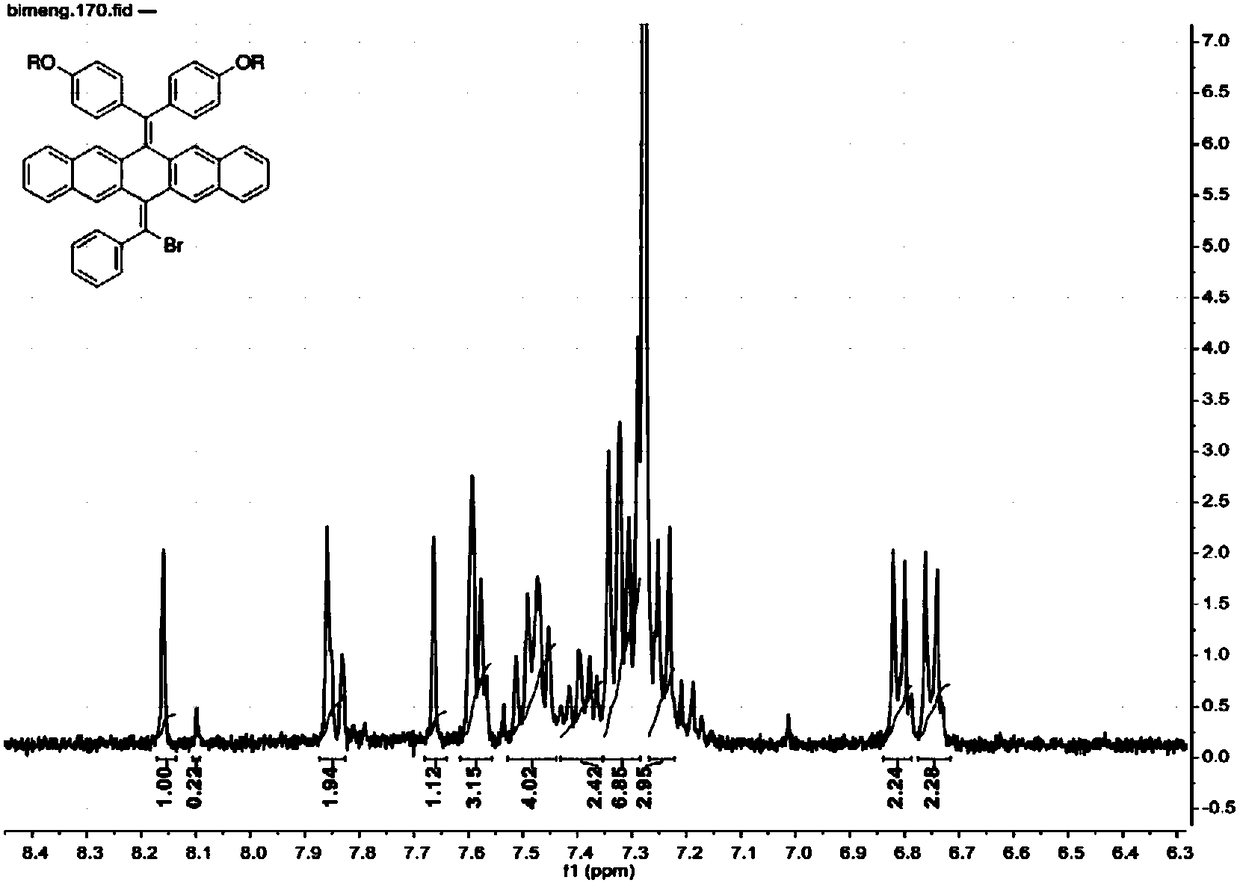
[0039] (2) Weigh compound 2 (1.00g, 1.02mmol), phenylboronic acid (0.064g, 0.52mmol), potassium carbonate K 2 CO 3 (1.44g, 10.40mmol) in a sealed tube, add 60mL of toluene, 12mL of ethanol, 12mL of mixed solvent composition of water, nitrogen gas and stir for 40min, then add the catalyst Pd...
Embodiment 3
[0043] The synthetic method of beach chair type nonacene adopts the following steps:
[0044] (1) Take CBr 4 Dissolve in toluene, stir for 30min under nitrogen gas, then add PPh 3 continue to keep N 2 Atmosphere, compound 1 was added after the color of the whole system turned light yellow. keep N 2 The atmosphere was heated to 80°C for 24h. After the reaction was completed, it was cooled to room temperature, and the reaction system was quickly suction-filtered, and the filtrate was spin-dried and then filtered through a fast silica gel column, and the filtrate was spin-dried to obtain compound 2.
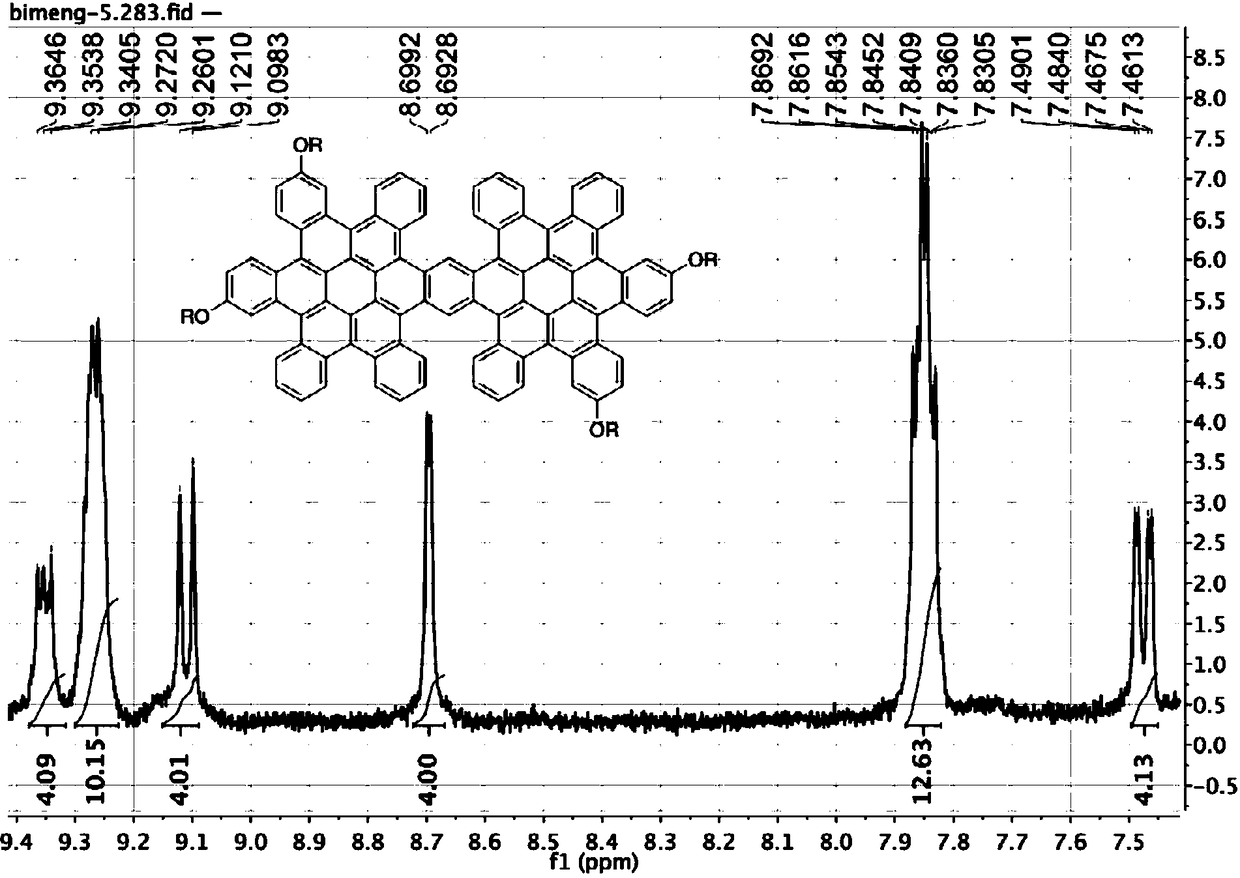
[0045] (2) Dissolve compound 2, phenylboronic acid, and potassium carbonate in a mixed solvent of toluene, ethanol, and water with a volume ratio of 5:1:1 after anaerobic treatment under a nitrogen atmosphere, and the catalyst Pd (PPh 3 ) 4 Compound 3 was obtained through a coupling reaction. The amount of catalyst used is 10 mol% of the total amount of raw materials, the rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com