Preparation method of red blood cell membrane-coated gelatin-loaded berberine hydrochloride gold nanoparticles and application thereof
A technology of berberine hydrochloride and red blood cell membrane, which is applied in the directions of medical preparations with non-active ingredients, medical preparations containing active ingredients, capsule delivery, etc. problem, to achieve the effect of high average drug loading, high average encapsulation efficiency and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
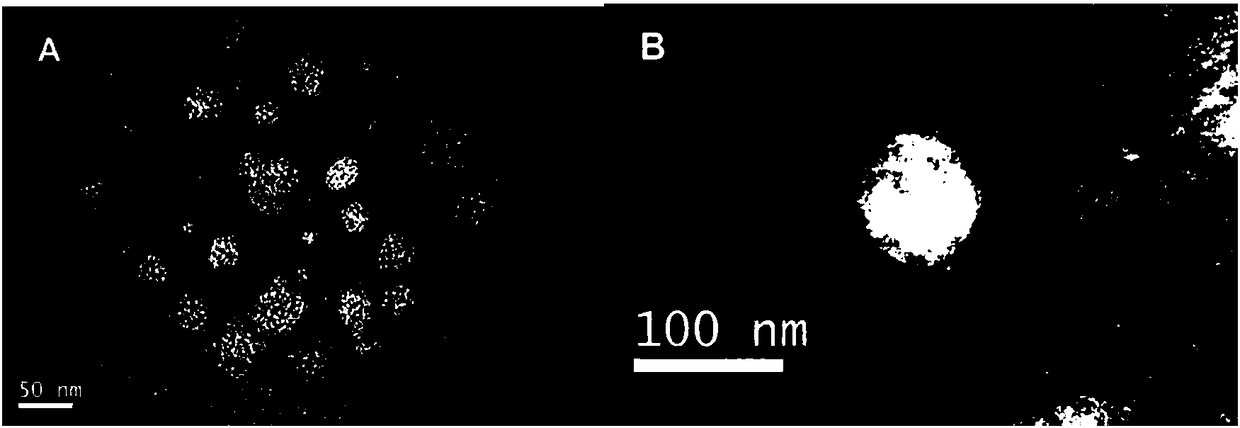
Image
Examples
Embodiment 1
[0064] Example 1 Preparation of erythrocyte membrane RBCM
[0065] (1) Get SD rats, weigh their body weight and record;
[0066] (2) Rats were injected intraperitoneally with 7% chloral hydrate solution, and the rats were anesthetized with an amount of 0.7mL / 100g until the turning reflex disappeared;
[0067] (3) After the rats were fully anesthetized, the rats were dissected to obtain blood by heart puncture, and the blood taken out was stored in an ice box;
[0068] (4) Take a 0.5mL EP tube, add 10μL of 5mg / mL sodium heparin solution to it, keep away from light, add 250μL of blood to each tube, and shake it to fully mix with the sodium heparin. Centrifuge at 3800rpm and 4°C for 10min;
[0069] (5) Remove the supernatant and white blood cell layer in (4), add 1mL 1×PBS, mix thoroughly, and centrifuge at 3800rpm, 4°C for 10min;
[0070] (6) Repeat (5) step 1 to 2 times;
[0071] (7) Remove the supernatant buffy coat in (6), add 900 μL 2mmol / L EDTA solution, mix well, then ...
Embodiment 2
[0075] Preparation of Example 2 RBCM-BH-GNPs
[0076] One, the preparation of the first group of preparations:
[0077] (1) Weigh gelatin solid particles (the gel strength of the gelatin is 250g Bloom), add ultrapure water, stir at room temperature for 30 minutes to swell, then heat to dissolve in a water bath at 50°C; filter the obtained solution and add 0.2 The NaOH solution of M adjusts the pH=6.0, and finally obtains a clear gelatin aqueous solution with a mass fraction of 0.5%;
[0078] (2) Take out a glutaraldehyde solution with a mass fraction of 50% under the storage condition of -20°C, and dissolve it at room temperature; add 4 times the volume of ultrapure water to the above solution, dilute it five times, and obtain a glutaraldehyde solution with a mass fraction of 10%. dialdehyde solution;
[0079] (3) Weigh the solid particles of sodium metabisulfite, add ultrapure water to dissolve, and obtain a sodium metabisulfite solution with a mass fraction of 1.6%, and st...
Embodiment 3
[0125] Example 3 Stability investigation of RBCM-BH-G NPs
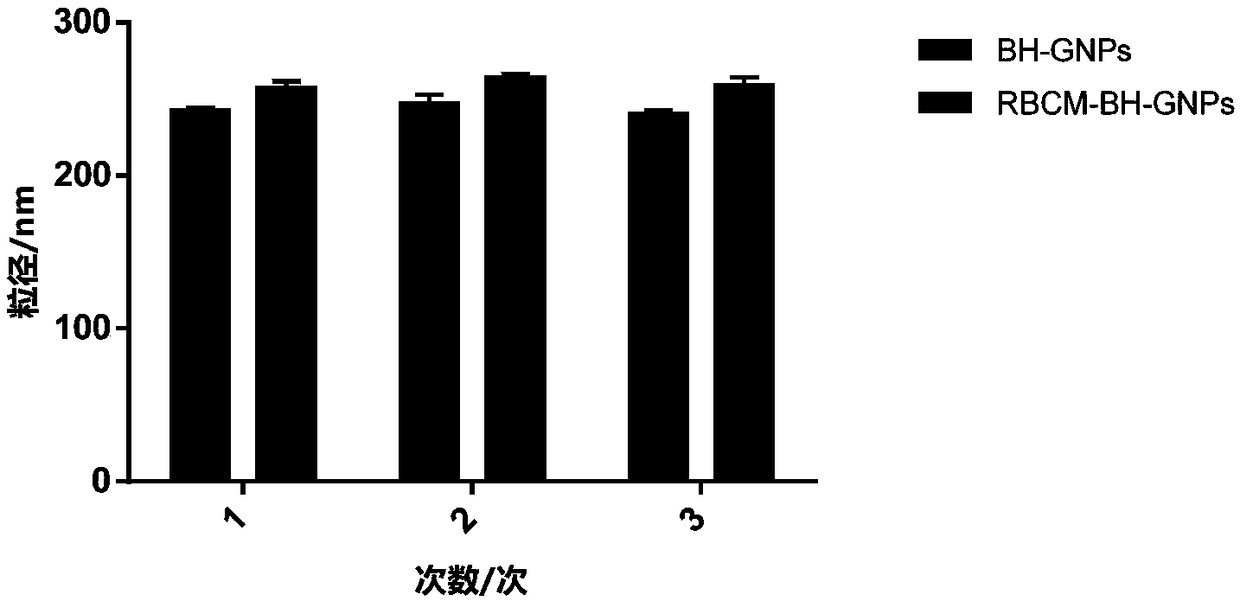
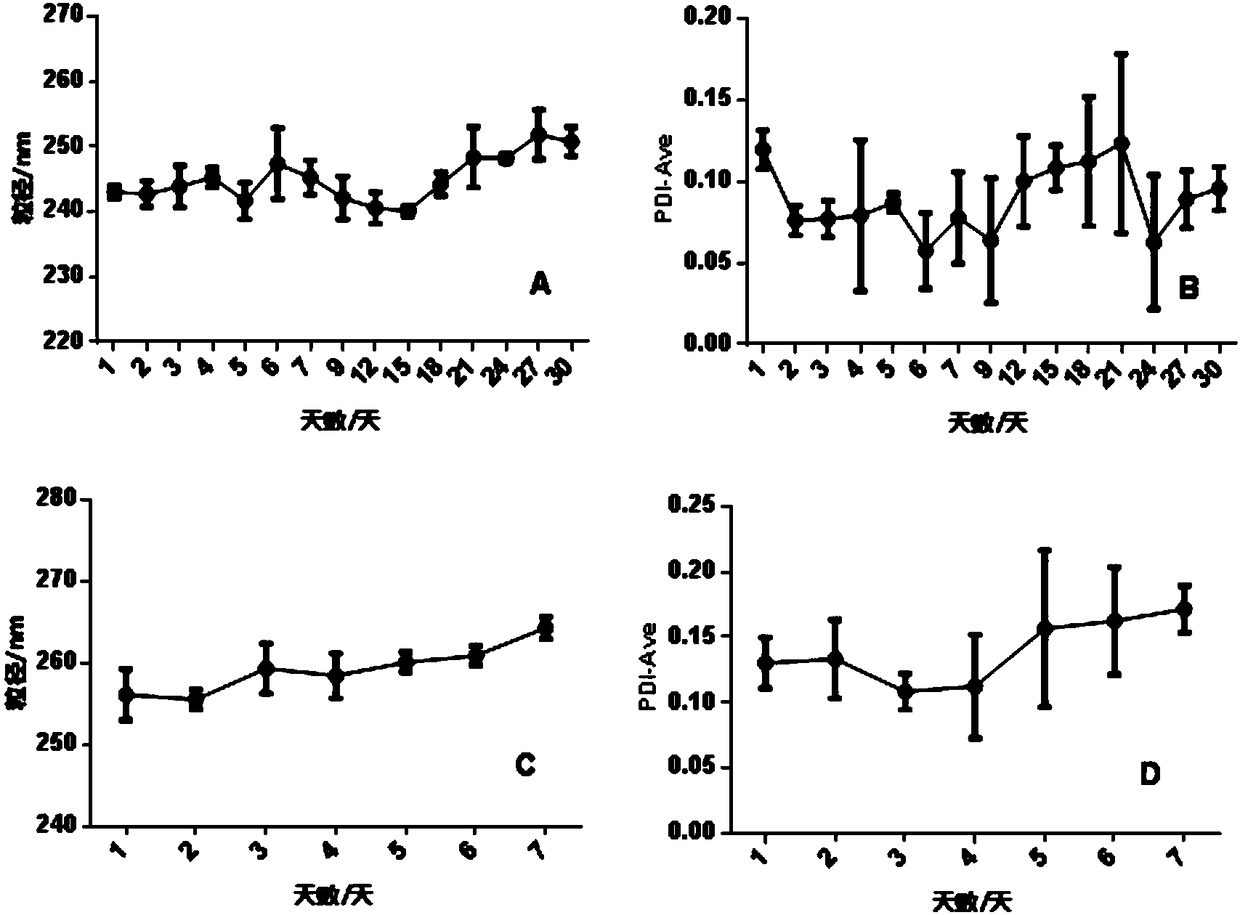
[0126] The prepared RBCM-BH-GNPs and BH-GNPs were stored at room temperature, and the particle size and polydispersity index were recorded continuously with a Malvern laser particle size analyzer. BH-GNPs were continuously measured for 30 days, and RBCM-BH-GNPs were measured for 7 days. particle stability. Depend on figure 2 It can be seen that the first group of preparations, BH GNPs (recorded as No. 1-1 preparations) have good stability within 30 days, and RBCM-BH-GNPs (recorded as No. 1-2 preparations) have good stability within 7 days. Stability, PDI remains below 0.15. Similarly, the second group of preparations, BH GNPs (recorded as No. 2-1 preparation) all had good stability within 30 days, and RBCM-BH-GNPs (recorded as No. 2-2 preparation) all had good stability within 7 days. Stability, PDI remains below 0.15. The third group of preparations, BH GNPs (recorded as No. 3-1 preparation) all have good stabil...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com