Vaccine adjuvant, vaccine composition, and use of sulfated chitooligosaccharide in preparation of vaccine adjuvant and vaccine composition
A vaccine composition and chitosan oligosaccharide technology, applied in the field of medicine, can solve the problems of serious adverse reactions of oil adjuvants, inflammation, ulcers and granulomas, and difficult metabolism of oil substances, and achieve good adjuvant effect and easy absorption , cost-effective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: preparation and physical and chemical properties of chitosan oligosaccharide
[0041] 1. Preparation of chitosan oligosaccharides:
[0042] Add deionized water into the batching tank, dissolve chitosan in 1% acetic acid solution, stir and heat up to 38±2.0°C. Add 5% (to the substrate) enzyme preparation, stir, and keep the temperature at 38°C. After the viscosity of the enzymolysis solution drops to 60%, start ultrafiltration (cut molecular weight at 6000). The part smaller than the cut-off molecular weight permeates the membrane, and the part larger than the cut-off molecular weight returns to the reactor to continue to degrade. The ultrafiltration permeate is passed through a nanomembrane filter (cutting molecular weight 300), and the permeated part is water, acetic acid and small molecule sugar (the permeated part can be recycled for preparing chitosan solution), and the non-permeated part is Concentrate into chitosan oligosaccharide solution and sp...
Embodiment 2
[0053] Embodiment 2: Preparation and physicochemical properties of sulfated chitosan oligosaccharide
[0054] 1. Preparation of 6-O-sulfated chitosan oligosaccharide (COSS):
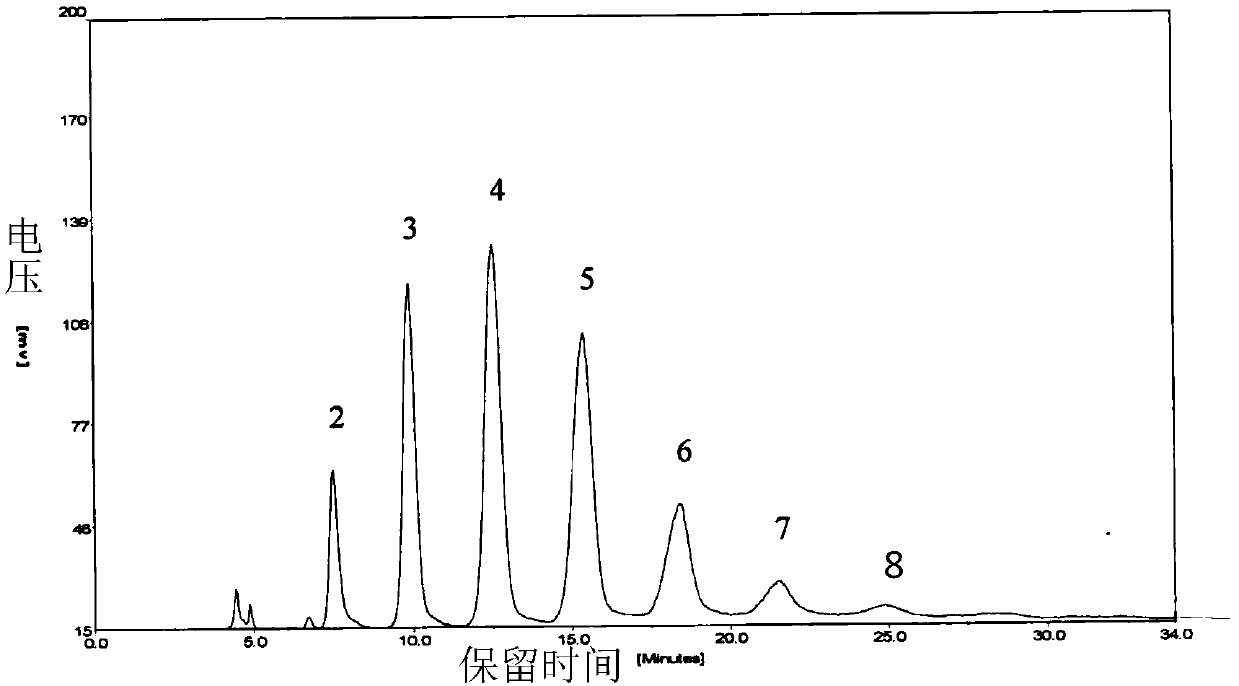
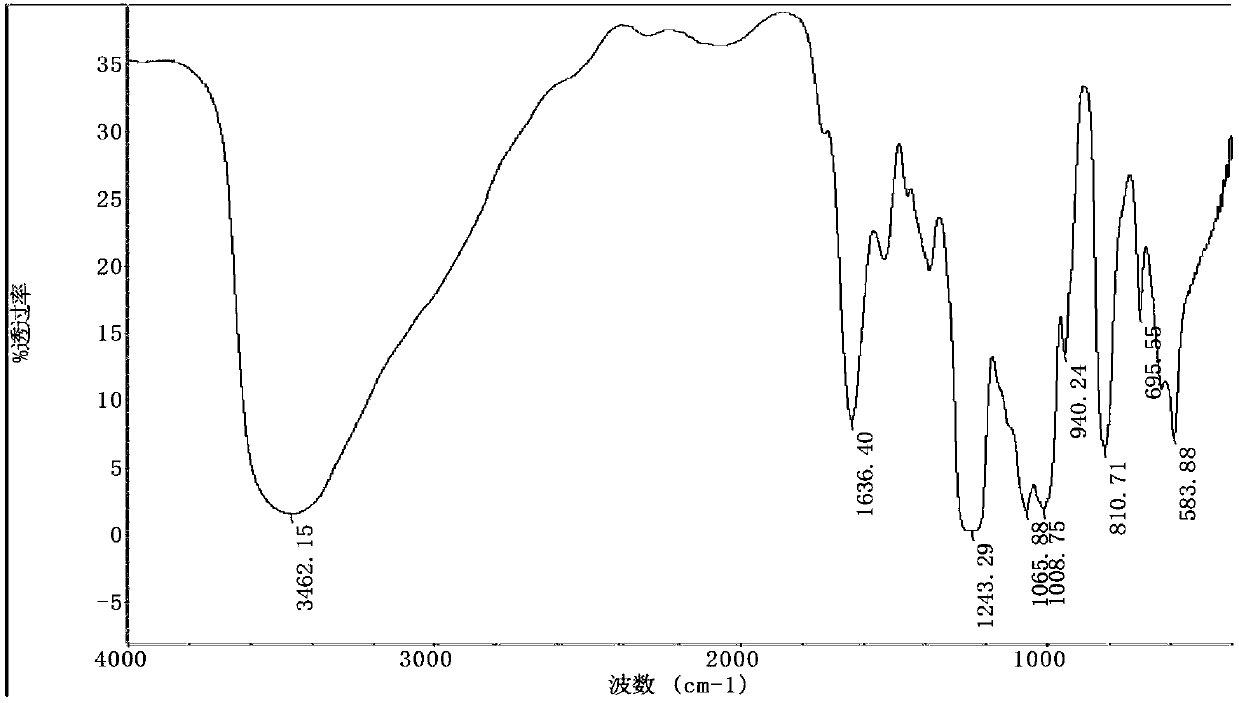
[0055] Concentrated sulfuric acid 20ml, HClSO3 10ml, chitosan oligosaccharide 500mg (504.3mg, 6.17mM), ice bath 30min, generate reddish-brown reaction solution, react at room temperature for 3h, absorb the reaction solution and add it to the Erlenmeyer flask equipped with pre-cooled ether solution, 4 Stand overnight at ℃, centrifuge at 10000rpm for 30min, wash the precipitate twice with 4℃ pre-cooled ether, centrifuge at 10000rpm for 30min, remove excess acid solution as much as possible, remove supernatant (add NaHCO3 powder to the supernatant solution to neutralize), and store in a fume hood Leave it to dry for 3 hours, dissolve the precipitate in 2ml of water, neutralize to neutral with 0.5M NaHCO3, and freeze-dry. The dried samples were desalted by Sephadex G15 column purification (Sephadex gel ch...
Embodiment 3
[0070] Example 3: Determination of adjuvant activity of sulfated oligochitosan to porcine reproductive and respiratory syndrome inactivated vaccine
[0071] The sulfated chitosan oligosaccharide in Example 2 was used as a vaccine adjuvant, and the inactivated porcine reproductive and respiratory syndrome vaccine (PRRSV) was used as an antigen, and the two were used in combination to immunize mice by intramuscular injection, and the antibody titer was determined. The specific method is as follows:
[0072] Experimental animals: BALb / c mice, 6-8 weeks old, 6 mice / group, female.
[0073] Drug concentration: oligochitosan (COS) prepared in the above-mentioned embodiment 1 and sulfated oligochitosan (COSS and SCOSS) prepared in embodiment 2: 4mg / ml; Porcine reproductive and respiratory syndrome inactivated vaccine (PRRSV , Guangdong Yongshun): 1.6mg / ml; ISA206 oil-emulsion adjuvant (French Seepic product): mixed with the vaccine at a volume ratio of 1:1.
[0074] Control solven...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com