Method for improving encapsulation yield of water-soluble drug microsphere drug-loading system
A technology for water-soluble drugs and drug-carrying systems, applied in the field of medicine, can solve the problems of obvious burst release phenomenon of microspheres, short in vitro release time, low drug encapsulation rate, etc., and achieves good fluidity, complete shape and stable preparation process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
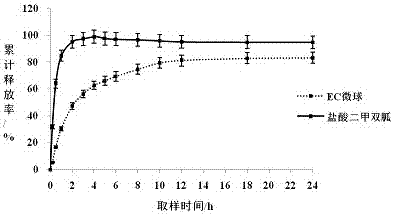
[0030] This example uses the method of the present invention to prepare microspheres whose carrier material is ethyl cellulose and the model drug metformin hydrochloride, and mainly investigates the influence of different gelatin concentrations on the encapsulation yield.
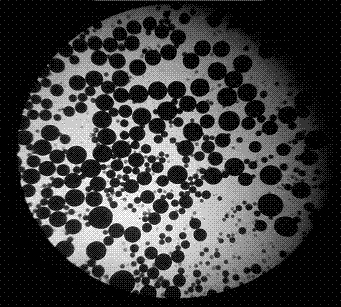
[0031] The drug concentration is 10%, and the oil phase is a dichloromethane solution saturated with metformin aqueous solution, wherein the concentration of ethyl cellulose is 10%, and the volume ratio of the inner water phase to the oil phase is 1:40. The water phase is 1% PVA, and its volume ratio to colostrum is 10:1, emulsified using a high-shear dispersing emulsifier. The gelatin concentrations were selected to be 0%, 15%, 20%, 25%, and 30%, respectively, and the encapsulation yield and drug loading of metformin hydrochloride microspheres were shown in Table 1. It can be seen that the encapsulation yield and drug loading are the highest when the gelatin concentration is 20%. figure 1 It is a 64-fold ...
Embodiment 2
[0035] In this example, the method of the present invention is used to prepare microspheres whose carrier material is PLGA and the model drug metformin hydrochloride.
[0036] The drug concentration is 10%, the oil phase adopts the dichloromethane solution saturated with metformin aqueous solution, wherein the PLGA concentration is 25%, the volume ratio of the inner water phase and the oil phase is 1:40, and the ultrasonic cell pulverizer is used for ultrasonication, and the outer water phase is 1% PVA, its volume ratio to colostrum is 10:1, emulsified using a high shear dispersing emulsifier. The gelatin concentration was selected as 20%, and the encapsulation yield and drug loading of metformin hydrochloride microspheres were 75.91% and 2.01%, respectively.
Embodiment 3
[0038] In this example, microspheres in which the carrier material is PLGA and the model drug is atropine sulfate are prepared by the method of the present invention.
[0039] The drug concentration is 10%, the oil phase adopts dichloromethane solution, wherein the PLGA concentration is 25%, the volume ratio of the inner water phase and the oil phase is 1:40, and the ultrasonic cell pulverizer is used for ultrasonication, and the outer water phase is 1%PVA. Its volume ratio to colostrum is 10:1, and it is emulsified using a high-shear dispersing emulsifier. When the concentration of gelatin is 20%, the encapsulation yield and drug loading of atropine sulfate microspheres are 77.36% and 3.65%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com