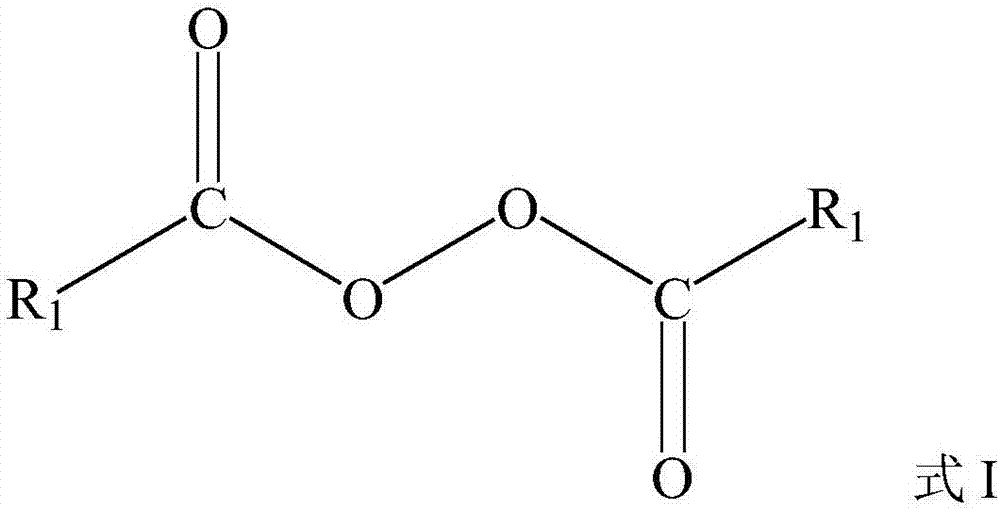
Preparation method of compound containing non-terminal-group double bonds
A compound, the technology of diacyl peroxide, applied in the preparation of organic compounds, the preparation of amino compounds and steroids from amines, etc., can solve the problems of hindering aliphatic peroxyacetyl compounds, difficult to control, etc., and achieve raw materials and catalysts. Inexpensive, efficient reaction, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059]
[0060] Add 58 μL (0.5 mmol) styrene 1-1, 600 mg (1.5 mmol,) lauroyl peroxide 1-2, 10 mol% catalyst Pd(OAc) to the reaction tube 2 , solvent toluene 2mL, add a magnetic stirrer, and then put it into a 90°C oil bath for 3 hours of reaction. After the reaction, cool to room temperature, dilute with ethyl acetate, filter with diatomaceous earth, evaporate and concentrate under reduced pressure to remove the solvent, the crude product is separated by column chromatography, the obtained product sample is recorded as 1-3, a total of 92.88mg, and the yield is 72% .
[0061] The NMR detection data of product samples 1-3 are as follows:
[0062] 1 H NMR (400MHz, CDCl 3 )δ7.34(d, J=7.2Hz, 2H), 7.28(t, J=7.2Hz, 2H), 7.18(t, J=7.2Hz, 1H), 6.37(d, J=15.8Hz, 1H) , 6.26-6.18 (m, 1H), 2.22-2.17 (m, 2H), 1.47-1.42 (m, 2H), 1.30-1.26 (m, 16H), 0.88 (t, J=6.8Hz, 3H).
[0063] 13 C NMR (100MHz, CDCl 3 )δ 137.99, 131.29, 129.69, 128.47, 126.74, 125.91, 33.09, 31.96, 29.71, 29.68,...
Embodiment 2
[0065]
[0066] Add 23 μL (0.2 mmol) styrene 2-1, 240 mg (0.6 mmol) lauroyl peroxide 2-2, 10 mol% catalyst Pd(TFA) to the reaction tube 2 , solvent tetrahydrofuran 1mL, add a magnetic stirrer, and then put it into a 90 ° C oil bath for 3 hours of reaction. After the reaction, cool to room temperature, dilute with ethyl acetate, filter with diatomaceous earth, and concentrate under reduced pressure to remove the solvent. The crude product is separated by column chromatography. .
[0067] The NMR detection data of product sample 2-3 are basically the same as the NMR detection data of product sample 1-3.
Embodiment 3
[0069]
[0070] Add 298.5 mg (0.75 mmol) lauroyl peroxide 3-2, 58 μL (0.5 mmol) styrene 3-1, 5 mol% catalyst Fe(OTf) to the reaction tube 3 , solvent tetrahydrofuran 2ml, add a magnetic stirrer, and then put it into a 60 ° C oil bath for 3 hours of reaction. After the reaction, cool to room temperature, dilute with ethyl acetate, filter with diatomaceous earth, and concentrate under reduced pressure to remove the solvent. The crude product is separated by column chromatography. .
[0071]The NMR detection data of the product sample 3-3 are basically the same as the NMR detection data of the product sample 1-3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com