Method for producing porcine pseudorabies gE gene deletion virus vaccine by using micro-carrier suspension culture cells
A technology of suspension culture and porcine pseudorabies, which is applied in the direction of microcarriers, biochemical equipment and methods, viruses, etc., can solve the problems of high price and increased cost of virus vaccines, increase the specific surface area, promote cell adherent growth, reduce The effect of production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Chitosan microcarrier preparation and performance testing
[0035] Component (weight)
Group A
Group B
Group C
Group D
Chitosan (g)
200
200
200
200
Polyhydroxyalkanoate (g)
60
70
80
/
[0036] Preparation of group A chitosan microcarriers:
[0037] Get chitosan 200g and be dissolved in the dilute acetic acid of 2% (v / v), magnetic stirring makes the chitosan sol solution that concentration is 12% (m / v), adds 3% (m / v) ammonium acetate, Stir evenly, add 60g of polyhydroxyalkanoate, ultrasonic treatment for 25min, then seal in a high-pressure steam boiler, treat at 250°C for 3.5h, cool to room temperature, centrifuge to collect the precipitate, wash with deionized water 3 times, at 60°C Under vacuum drying 5h, obtain the chitosan microsphere that the surface contains polyhydroxyalkanoate, soak the chitosan microsphere that obtains in 1% (m / v) glutaraldehyde solution, react 2h under normal temperat...
Embodiment 2
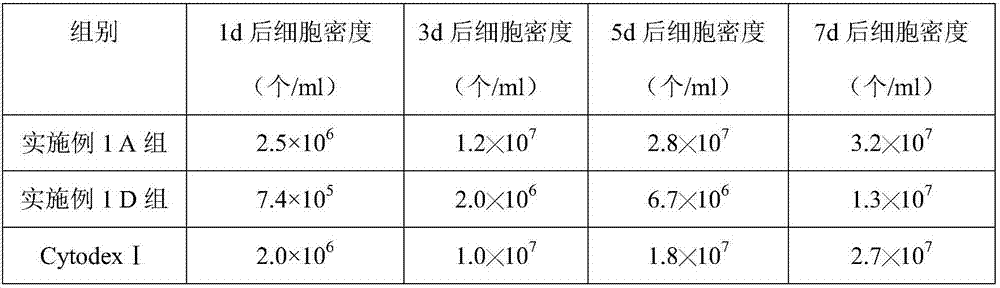
[0045] Embodiment 2 The effect of chitosan microcarrier on the growth of BHK-21 cells
[0046] The chitosan microcarriers prepared in Example 1A and Group D and the commercially available Cytodex I microcarriers were used to carry out suspension culture of BHK21 cells, specifically:
[0047] (1) Take the BHK21 cell species out of the liquid nitrogen tank for recovery, add it to DMEM medium containing 10% newborn bovine serum, and store it at 37°C, 5% CO 2 cultured until it grows into a good single layer, then digested with an appropriate amount of trypsin digestion solution containing 0.02% EDTA, digested at 37°C for 6min, and adjusted the cell density to 4.5×10 with DMEM medium containing 10% newborn bovine serum. 5 cell suspension per mL;
[0048] (2) Inoculate the cell suspension obtained in step (1) into a stirred bioreactor, using 1.5% (m / v) FBS, 1% (m / v) D-glucosamine, 3.5mmol / L glutamine The DMEM culture fluid of amide, 6% (m / v) growth promoter, 1.0% (v / v) double anti...
Embodiment 3
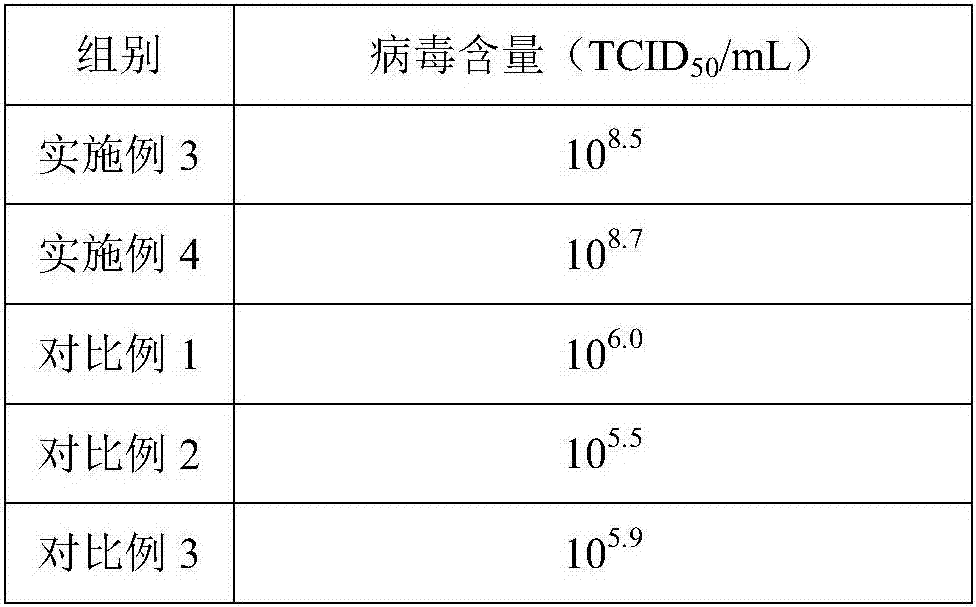
[0053] Example 3 Production of porcine pseudorabies gE gene deletion virus by microcarrier suspension culture BHK-21 cells
[0054] (1) Take the BHK21 cell species out of the liquid nitrogen tank for recovery, add it to DMEM medium containing 10% newborn bovine serum, and store it at 37°C, 5% CO 2 cultured until it grows into a good single layer, then digested with an appropriate amount of trypsin digestion solution containing 0.02% EDTA, digested at 37°C for 6min, and adjusted the cell density to 4.5×10 with DMEM medium containing 10% newborn bovine serum. 5 cell suspension per mL;
[0055] (2) Inoculate the cell suspension obtained in step (1) into a stirred bioreactor, using 1.5% (m / v) FBS, 1% (m / v) D-glucosamine, 3.5mmol / L glutamine The DMEM culture fluid of amide, 6% (m / v) growth promoter, 1.0% (v / v) double antibody carries out microcarrier suspension culture, wherein, described growth promoter is made of keratan sulfate oligosaccharide and active mineral The yeast poly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com