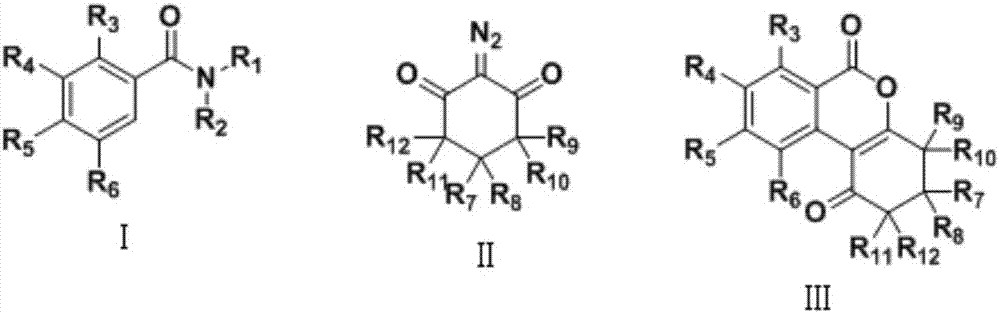
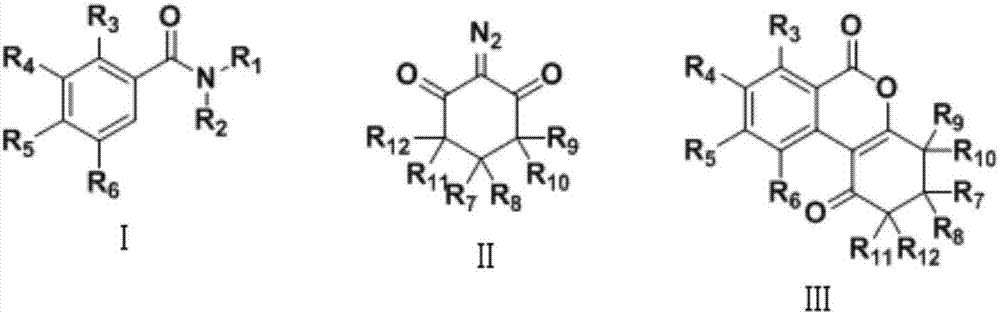
Preparation method of isocoumarin derivative
A technology for isocoumarin and derivatives, which is applied in the field of preparation of isocoumarin derivatives, can solve the problems of difficulty in introducing five-membered rings or six-membered rings, poor thermal stability of peroxides, and reaction uncertainty.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
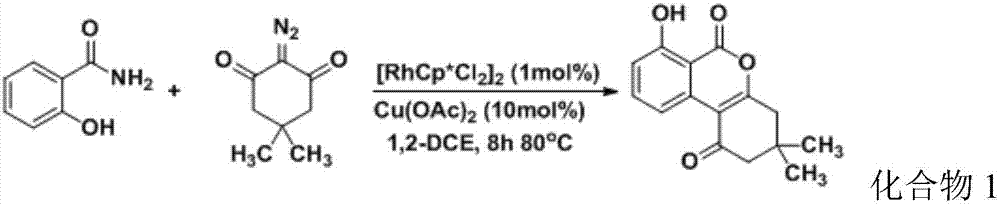
[0042] To the 25mL reaction flask, add [RhCp*Cl 2 ] 2 (0.01mmol), Cu(OAc) 2 (0.1mmol), salicylamide (1mmol), 5,5-dimethyl-2-diazo-1,3-cyclohexanedione (1mmol), 1,2-dichloroethane (1,2- DCE) (5mL), reacted at 80°C for 8h, and tracked the reaction by thin layer chromatography (TLC). After the reaction, extracted with 15mL×2 dichloromethane and 10mL saturated brine, combined the organic phases, and added anhydrous sulfuric acid Sodium drying for 6h column chromatography (developing agent petroleum ether / ethyl acetate v / v=6:1) can give white product, the product is carried out 1 H NMR and 13 C NMR detects, and obtained result is as follows:
[0043] 1 H NMR (300MHz CDCl 3 )δ: 10.87(s, 1H), 8.47(d, J=8.1Hz, 1H), 7.68(t, J=8.1Hz, 1H), 7.01(d, J=8.4Hz, 1H), 2.78(s, 2H), 2.51(s, 2H), 1.17(s, 6H). 13 C NMR (75MHz CDCl 3 )δ: 196.4, 166.9, 164.7, 161.7, 138.4, 133.7, 116.5, 115.9, 111.3, 105.3, 52.8, 42.2, 31.8, 28.1. Depend on 1 The hydroxyl peak at 10.87 in H NMR and 13 The...
Embodiment 2
[0046] In addition to salicylamide, 5,5-dimethyl-2-diazo-1,3-cyclohexanedione, [RhCp * Cl 2 ] 2 Except that the amount of copper acetate added is 1mmol, 0.9mmol, 0.01mmol, 0.08mmol respectively, other conditions and steps are the same as in Example 1, the white product can be obtained, and the product is carried out 1 H NMR and 13 C NMR detection, the obtained result is the same as the detection result of compound 1 in Example 1, which is confirmed to be compound 1, and the yield of compound 1 is calculated to be 81%.
Embodiment 3
[0048] In addition to salicylamide, 5,5-dimethyl-2-diazo-1,3-cyclohexanedione, [RhCp * Cl 2 ] 2 Except that the amount of copper acetate added is 1mmol, 1.1mmol, 0.03mmol, 0.12mmol respectively, other conditions and steps are the same as in Example 1, the white product can be obtained, and the product is carried out 1 H NMR and 13 C NMR detection, the obtained result is the same as the detection result of compound 1 in Example 1, which is confirmed to be compound 1, and the yield of compound 1 is calculated to be 84%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com