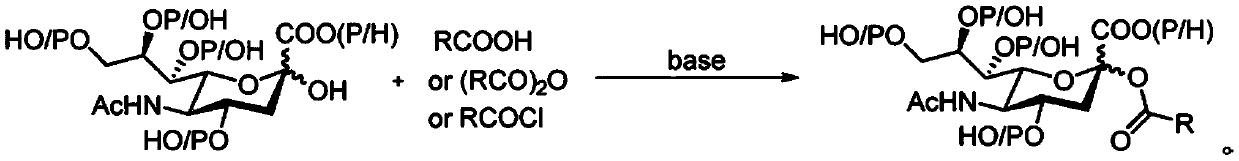
Sialic acid-carboxylic acid compound conjugate and preparation method thereof
A compound and carboxylic acid technology, applied in the field of medicinal chemistry, can solve the problems of uncontrollable product stereoselectivity, cumbersome operation, and low synthesis efficiency, and achieve the effects of high synthesis efficiency, simple operation, and reduced irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A kind of preparation method of sialic acid-acetic acid conjugate, comprises the steps:
[0036] (1) Weigh 134mg (0.2mmol) of fully acetyl-protected sialyl glucosinolate donor 1 and 170mg of newly activated Add MS to the reaction flask, add 4 mL redistilled dichloromethane to dissolve, stir at 25°C under the protection of argon atmosphere, add 34 μL (0.6 mmol) acetic acid 2a, and slowly stir at 25°C for 15 minutes.
[0037] (2) Quickly add 50 μL (0.4 mmol) of boron trifluoride diethyl ether and 90 mg (0.4 mmol) of iodosuccinimide to the reaction system of step (1), and the color of the solution will change from colorless and turbid to opaque Dark purple, stirred at 25°C for 5 minutes to react.
[0038] (3) After the reaction of step (2) finishes, the reaction system is suction filtered, and the filtrate is diluted with organic solvent methylene chloride, and the organic phase is saturated with sodium thiosulfate (Na 2 S 2 o 3 ) solution was washed once, the aqueous...
Embodiment 2
[0042] A preparation method of sialic acid-n-hexanoic acid conjugate, comprising the steps of:
[0043] (1) Weigh 134mg (0.2mmol) of fully acetyl-protected sialyl glucosinolate donor 1 and 170mg of newly activated Add MS into the reaction flask, add 4 mL redistilled dichloromethane to dissolve, under the protection of argon atmosphere, stir at 25 °C, add 75 μL (0.6 mmol) n-hexanoic acid 2b, and slowly stir at 25 °C for 15 minutes.
[0044] (2) (with embodiment 1).
[0045] (3) After the reaction of step (2) finishes, the reaction system is suction filtered, and the filtrate is diluted with organic solvent methylene chloride, and the organic phase is saturated with sodium thiosulfate (Na 2 S 2 o 3 ) solution was washed once, the aqueous phase was extracted three times with organic solvent dichloromethane, then the organic phases were combined, dried with anhydrous sodium sulfate (Na2SO4), filtered and spin-dried, and eluted through silica gel column chromatography (petroleu...
Embodiment 3
[0049] A preparation method of sialic acid-5-hexynoic acid conjugate, comprising the steps of:
[0050] (1) Weigh 134mg (0.2mmol) of fully acetyl-protected sialyl glucosinolate donor 1 and 170mg of newly activated Add MS to the reaction flask, add 4 mL redistilled dichloromethane to dissolve, stir at 25°C under argon atmosphere, add 66 μL (0.6 mmol) 5-hexynoic acid 2c, and slowly stir at 25°C for 15 minutes.
[0051] (2) (with embodiment 1).
[0052] (3) After the reaction of step (2) finishes, the reaction system is suction filtered, and the filtrate is diluted with organic solvent methylene chloride, and the organic phase is saturated with sodium thiosulfate (Na 2 S 2 o 3 ) solution was washed once, the aqueous phase was extracted three times with organic solvent dichloromethane, then the combined organic phases were washed with anhydrous sodium sulfate (Na 2 SO 4 ), filtered and spin-dried, and eluted by silica gel column chromatography (ratio of petroleum ether to et...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com