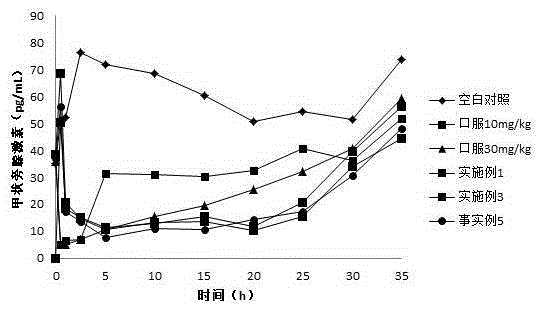
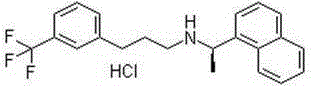
Skin external-use patch containing calcium-sensing receptor stimulant
A calcium-sensitive receptor and agonist technology, applied in the field of pharmaceutical preparations, to avoid the first-pass effect of the liver, good safety, and reduce toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] prescription composition
[0027] materials Amount added (g) percentage% Cinacalcet hydrochloride 2.5 1.39 active vitamin D 0.2 0.11 SIS 84.3 46.83 liquid paraffin 47.8 26.55 polymer polyisobutylene 10.0 5.56 Low Molecular Polyisobutylene 10.0 5.56 Terpene resin 18.0 10.00 Menthol 0.9 0.50 Azone 0.9 0.50 Butylated hydroxytoluene 3.6 2.00 Zinc stearate 1.8 1.00 batch 100 stickers
[0028] Preparation method: heat and stir SIS, part of liquid paraffin, high-molecular polyisobutylene, low-molecular polyisobutylene, terpene resin, and dibutyl hydroxytoluene; add menthol and Azone to the above liquid and mix evenly; add hard After zinc fatty acid and part of liquid paraffin are mixed evenly, add them to the above liquid and stir evenly; after the paste cools down, add the prescribed amount of cinacalcet hydrochloride and active vitamin D and stir evenly; Clothed on the pro...
Embodiment 2
[0030] prescription composition
[0031] materials Amount added (g) percentage% Cinacalcet hydrochloride 10.0 5.56 Vitamin D 5.0 2.78 SIS 61.3 34.06 liquid paraffin 37.6 20.89 hydrogenated petroleum resin 63.0 35.00 Azone 0.4 0.21 Butylated hydroxytoluene 1.8 1.00 Titanium dioxide 0.9 0.50 batch 100 stickers
[0032] Preparation method: Heat and dissolve SIS, part of liquid paraffin, hydrogenated petroleum resin, and dibutyl hydroxytoluene in the prescribed amount, and mix well; add Azone to the above mixed liquid; mix the remaining liquid paraffin and titanium dioxide evenly, and then add to the above solution Middle; after the ointment cools down, add the prescribed amount of cinacalcet hydrochloride and vitamin D and stir evenly, apply the obtained medicated ointment evenly on the protective layer, and after laminating with the backing layer as the preparation, cut into a set size and shape, ...
Embodiment 3
[0034] Prescription composition:
[0035] materials Amount added (g) percentage% Cinacalcet hydrochloride 5.0 5.56 SIS 34.0 37.78 liquid paraffin 19.7 21.89 Polybutene 4.5 5.00 Terpene resin 10.8 12.00 Hydrogenated Rosin Glycerides 13.5 15.00 Menthol 0.3 0.33 Oleic acid 0.1 0.11 Avobenzone 1.8 2.00 Zinc stearate 0.3 0.33 batch 100 stickers
[0036] Preparation method: heat and dissolve SIS, part of liquid paraffin, polybutene, terpene resin, hydrogenated rosin glyceride, and avobenzone in the prescribed amount, and mix evenly; add menthol and oleic acid to the above mixed liquid; The remaining liquid paraffin and zinc stearate are mixed evenly and then added to the above solution; after the paste is cooled down, the prescribed amount of cinacalcet hydrochloride is added and stirred evenly, and the obtained medicated paste is evenly coated on the protective layer, After laminating w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com