Cordycepin nanometer liposome, preparation method, and antitumor activity applications thereof
A nano-liposome and cordycepin technology, which is applied in the direction of anti-tumor drugs, liposome delivery, organic active ingredients, etc., can solve the problem that the encapsulation rate of liposomes can only be reached, but the encapsulation rate of liposomes cannot be reached, and there is no further progress Issues such as the verification of the activity of cordycepin liposomes in vivo, to achieve short half-life, inhibit the growth of tumors, and facilitate absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of cordycepin liposomes whose phospholipid is hydrogenated soybean lecithin by pH gradient method or reverse evaporation method
[0027]
[0028] Preparation method: (1) pH gradient method
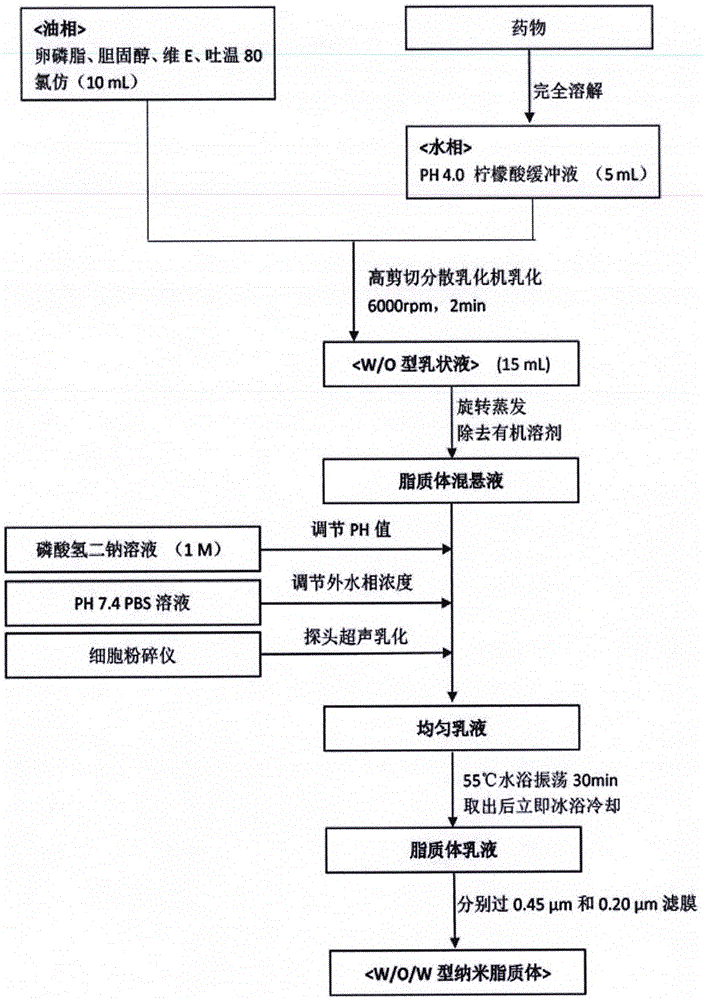
[0029] ① Take the prescribed amount of lecithin, cholesterol, Tween 80 and V E Dissolve in 10ml of chloroform to form a clear solution, and remove the chloroform by rotary evaporation under reduced pressure in a 45°C water bath to form a uniform lipid film.
[0030]② Add 10ml of pH4.0 citric acid buffer, hydrate in a 45°C water bath under normal pressure for 20 minutes, and then hydrate with magnetic stirring in a 45°C water bath for 1 hour. Ultrasonic the probe for 70 seconds (power 360W, super 3 seconds stop 4 second), and then pass through the microporous membrane method of 0.45 μm and 0.22 μm respectively to size the particles to obtain blank liposomes.
[0031] ③ Add the cordycepin solution, adjust the pH value to 7-7.4 with 1mol / L disodium hydrogen phosphate s...
Embodiment 2
[0036] Cordycepin liposomes whose phospholipid is hydrogenated soybean lecithin were prepared by the combination of "PH gradient method-reverse evaporation method"
[0037]
[0038]
[0039] Preparation method: ① Combine lecithin, cholesterol, Tween 80, V E The fat-soluble components are dissolved in 10ml of chloroform to form a clear solution.
[0040] ② Dissolve cordycepin in 5ml of citric acid buffer solution with pH 4.0, slowly add to the oil phase, and shear emulsify until a stable W / O emulsion is formed.
[0041] ③Place the emulsion in a 37°C water bath to remove the chloroform by rotary evaporation under reduced pressure, and form a gel on the bottle wall (a little PBS solution with pH 7.4 can be added), and continue the rotary evaporation to make the gel on the bottle wall fall off.
[0042] ④ 1mol / L disodium hydrogen phosphate solution to adjust the pH value of the external water phase to about 7.0, and PBS buffer solution with pH 7.4 to adjust the concentratio...
Embodiment 3
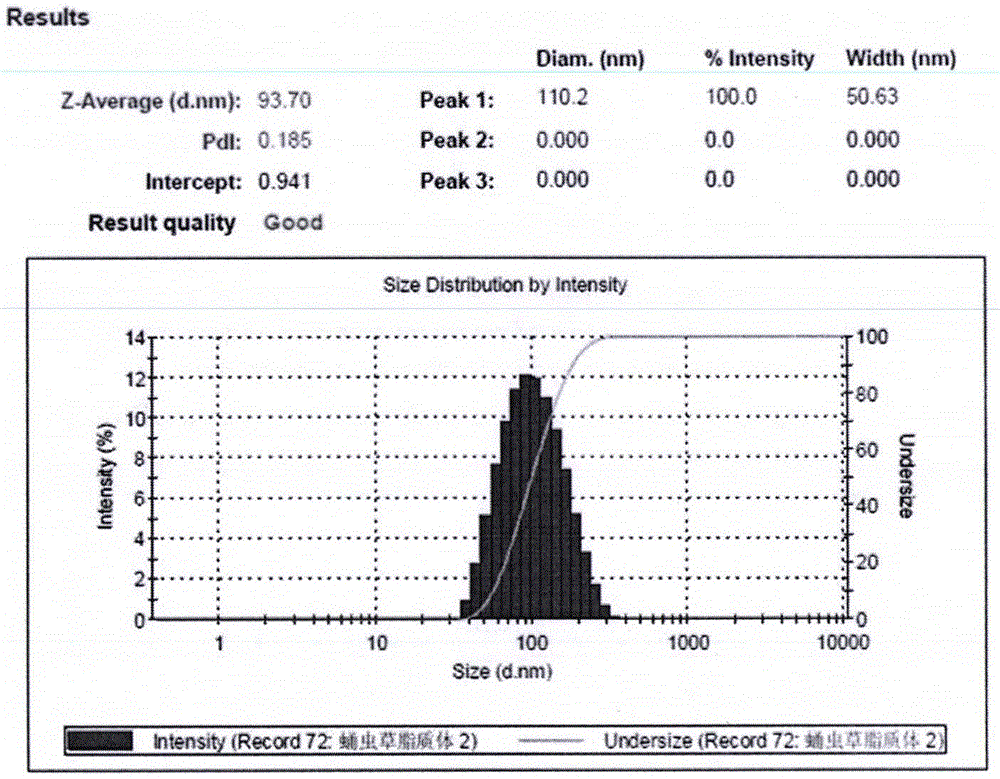
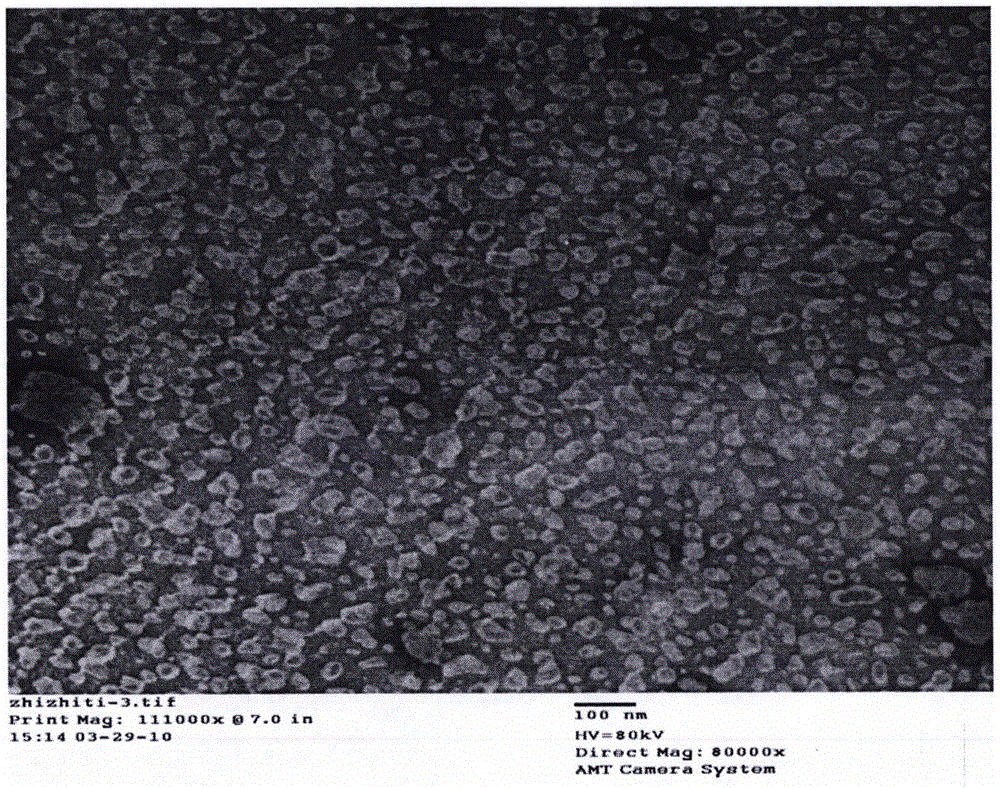
[0045] Determination of Liposome Encapsulation Efficiency
[0046] The method used Sephadex gel method, the specific steps are as follows:
[0047] 1. Take 0.5ml of the prepared cordycepin liposome, slowly add it to the pre-saturated Sephadex G-25 column, elute with normal saline at a flow rate of 0.9ml / min, collect the free drug part, and repeat the collection for three times. The parts were recorded as sample 1, sample 2, and sample 3, and the content of cordycepin in the free drug eluent was determined by high performance liquid chromatography. In addition, take 0.125ml of cordycepin liposome, demulsify with 0.875ml of methanol, and pass through a 0.45μm organic filter to obtain a sample, which is recorded as the total sample. After liquid phase quantitative determination, 4 times its value is regarded as 0.5ml lipid The total cordycepin content in the body.
[0048] 2. Liquid chromatography conditions for determination of cordycepin content
[0049] Instrument: Agilent ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com