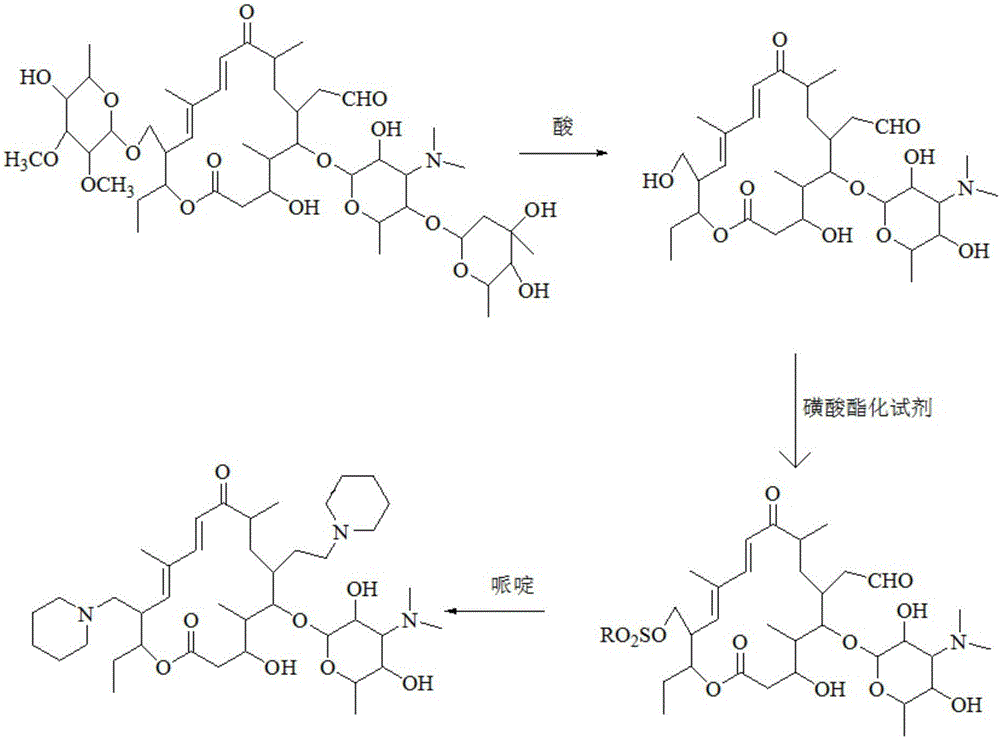
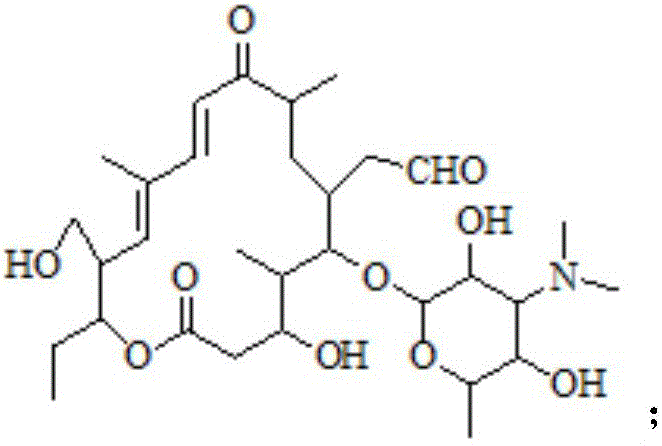
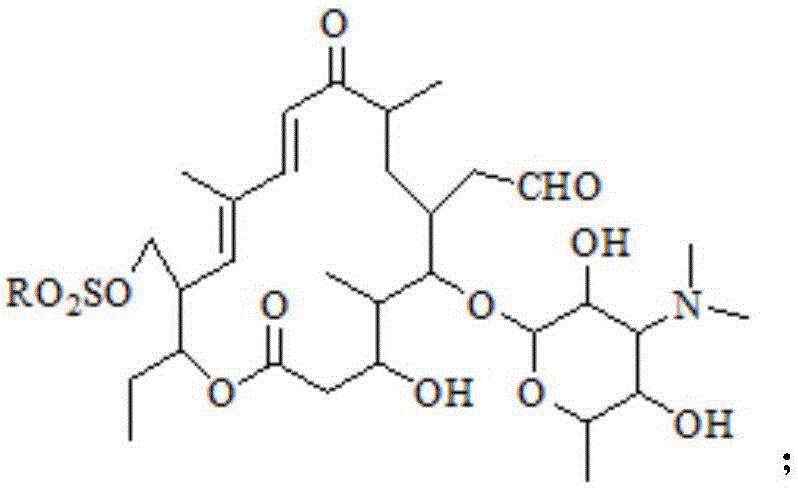
Preparation method of tildipirosin
A technology of tylosin and tylosin, which is applied to the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve unfavorable industrial scale-up production, incomplete silanization protection, and prone to side reactions, etc. problem, to achieve the effect of low cost, simple and easy operation, and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Preparation of hydrolyzate
[0034] Dissolve 40g of tylosin tartrate in 100mL of water, add 110g of hydrochloric acid with a mass fraction of 20%, raise the temperature to 55°C and keep it warm for 3 hours. After the liquid phase monitors that the reaction is complete, cool down to room temperature and add 250mL of dichloromethane to the reaction solution , adjust the pH to 9-10 with 30% sodium hydroxide solution under ice-bath conditions, extract and separate the layers, and wash the organic layer three times with 300 mL water respectively.
[0035] (2) Preparation of sulfonated product
[0036] Take the organic layer of the previous reaction, add 8g p-toluenesulfonyl chloride and 9g triethylamine respectively, raise the temperature to reflux state and keep it warm for 3 hours, after the liquid phase monitors the reaction is complete, cool down to room temperature and add 200mL saturated sodium bicarbonate, extract and separate layers, After the organic layer was ...
Embodiment 2
[0040] (1) Preparation of hydrolyzate
[0041] Dissolve 40g of tylosin tartrate in 100mL of water, add 145g of hydrochloric acid with a mass fraction of 20%, raise the temperature to 60°C and keep it warm for 1.5 hours. After the liquid phase monitors that the reaction is complete, cool down to room temperature and add 250mL of ethyl acetate to the reaction solution , adjust the pH to 9-10 with 30% sodium hydroxide solution under ice-bath conditions, extract and separate the layers, and wash the organic layer three times with 300 mL water respectively.
[0042] (2) Preparation of sulfonated product
[0043]Take the organic layer of the reaction in the previous step, add 7.5g p-toluenesulfonyl chloride and 8.1g triethylamine respectively, raise the temperature to 50°C and keep it warm for 1.5 hours. After the organic layer was washed three times with 250 mL of water, the organic solvent was removed by rotary evaporation to obtain a total of 22.8 g of light yellow solid.
[00...
Embodiment 3
[0047] (1) Preparation of hydrolyzate
[0048] Dissolve 40g of tylosin tartrate in 100mL of water, add 130g of hydrochloric acid with a mass fraction of 20%, raise the temperature to 55°C and keep it warm for 2 hours. After the liquid phase monitors that the reaction is complete, cool down to room temperature and add 250mL of dichloromethane to the reaction solution , adjust the pH to 9-10 with 30% sodium hydroxide solution under ice-bath conditions, extract and separate the layers, and wash the organic layer three times with 300 mL water respectively.
[0049] (2) Preparation of sulfonated product
[0050] Take the organic layer of the previous step reaction, add 8.4g p-toluenesulfonyl chloride and 7.6g triethylamine respectively, raise the temperature to reflux state and keep it warm for 2.5 hours, after the liquid phase monitoring reaction is complete, cool down to room temperature and add 200mL saturated sodium bicarbonate, extract After the organic layer was washed three...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com