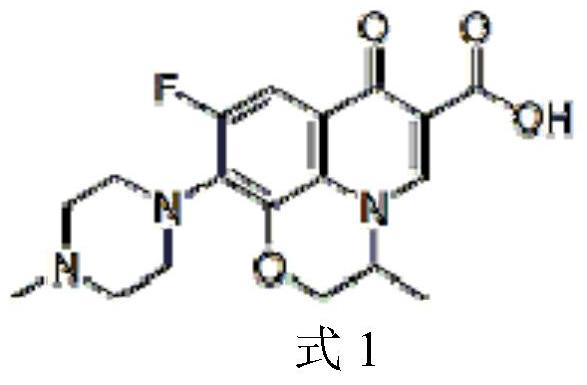
Method for separating ofloxacin enantiomers
A technology of enantiomers and ofloxacin, which is applied in the field of pharmacy, can solve problems such as the technical method of splitting and preparing ofloxacin that has not been seen, and achieve stable drug quality, full recycling, and good stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
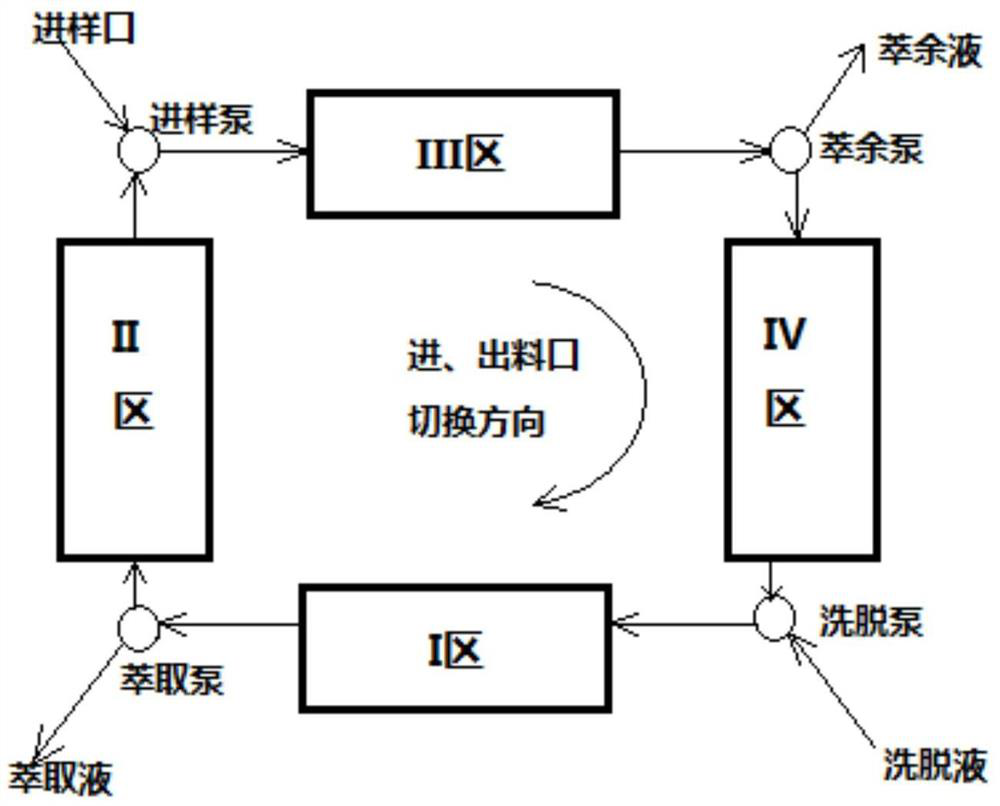
Image
Examples
Embodiment 1
[0045] Mobile phase: ethanol: n-hexane: phosphoric acid = 95:5:0.5
[0046] Flow rate: 1ml / min
[0047] Injection concentration: ofloxacin racemate: 0.2mg / ml
[0048] Injection liquid flow rate: V1=0.1ml / min
[0049] Mobile phase flow rate: V2=1.0ml / min
[0050]Flushing fluid flow rate: V3=2.0ml / min
[0051] F pump pressure: 6.8MPa
[0052] P pump pressure: 7.5MPa
[0053] D pump pressure: 6.8MPa
[0054] Switching time: 8.5min
[0055] Column temperature: 25°C
[0056] Finished product analysis
[0057] The composition of the extract and raffinate was analyzed by chiral column Chiralcel OD-H, wherein the contents of levofloxacin or dextrofloxacin were 100% and 98%, respectively.
Embodiment 2
[0059] Mobile phase: ethanol: n-hexane: phosphoric acid = 85:15:1
[0060] Flow rate: 1ml / min
[0061] Injection concentration: ofloxacin racemate: 0.2mg / ml
[0062] Injection liquid flow rate: V1=0.1ml / min
[0063] Mobile phase flow rate: V2=1.0ml / min
[0064] Washing liquid flow rate: V3=0.5ml / min
[0065] F pump pressure: 6.5MPa
[0066] P pump pressure: 4.5MPa
[0067] D pump pressure: 6.5MPa
[0068] Switching time: 8.5min
[0069] Column temperature: 25°C
[0070] Finished product analysis
[0071] The composition of the extract and raffinate was analyzed by chiral column Chiralcel OD-H, wherein the content of levofloxacin or dextrofloxacin was 90% and 100%, respectively.
Embodiment 3
[0073] Mobile phase: ethanol: n-hexane: phosphoric acid = 90: 10: 0.5
[0074] Flow rate: 1ml / min
[0075] Injection concentration: ofloxacin racemate: 0.2mg / ml
[0076] Injection liquid flow rate: V1=0.1ml / min
[0077] Mobile phase flow rate: V2=1.0ml / min
[0078] Flushing fluid flow rate: V3=2.0ml / min
[0079] F pump pressure: 6.8MPa
[0080] P pump pressure: 7.5MPa
[0081] D pump pressure: 7.0MPa
[0082] Switching time: 8.5min
[0083] Column temperature: 25°C
[0084] Finished product analysis
[0085] The composition of the extract and raffinate was analyzed by chiral column Chiralcel OD-H, wherein the contents of levofloxacin or dextrofloxacin were 100% and 90%, respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com