Catalyst for splitting racemic epoxyalkane and application thereof
A technology for alkylene oxide and catalyst, which is applied in the field of catalyst for splitting racemic alkylene oxide, can solve the problems of high dosage, not very ideal, etc., and achieves the effects of simple method, reduced operating cost and reduced waste.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The resolution reaction of the model system with 1,2-epoxyhexane and trimethylsilazide as reactants was carried out.
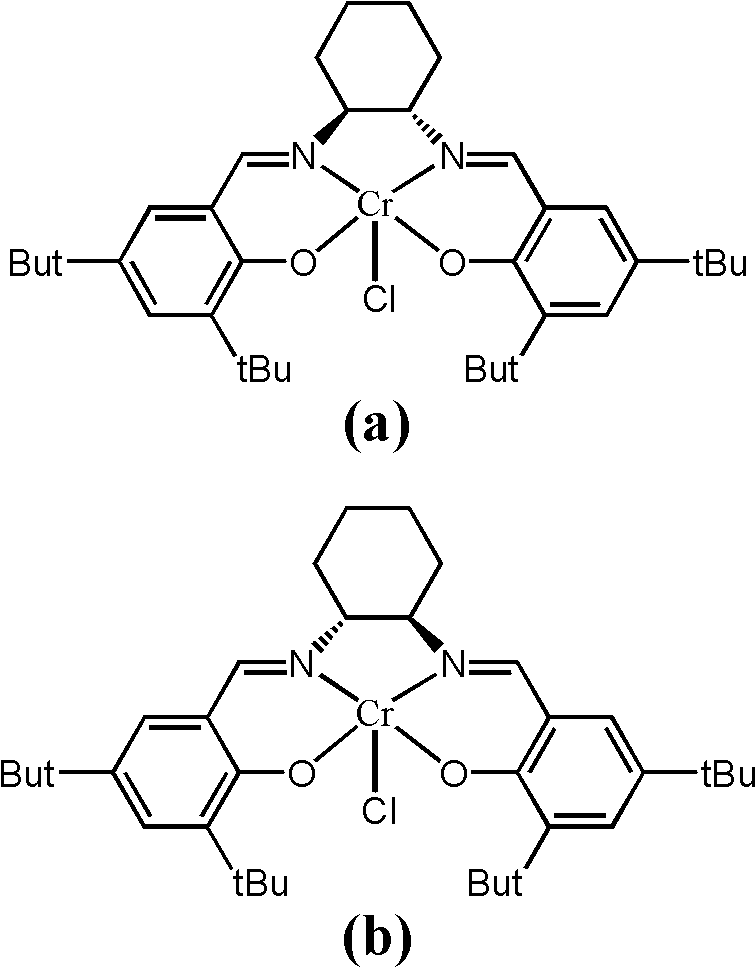
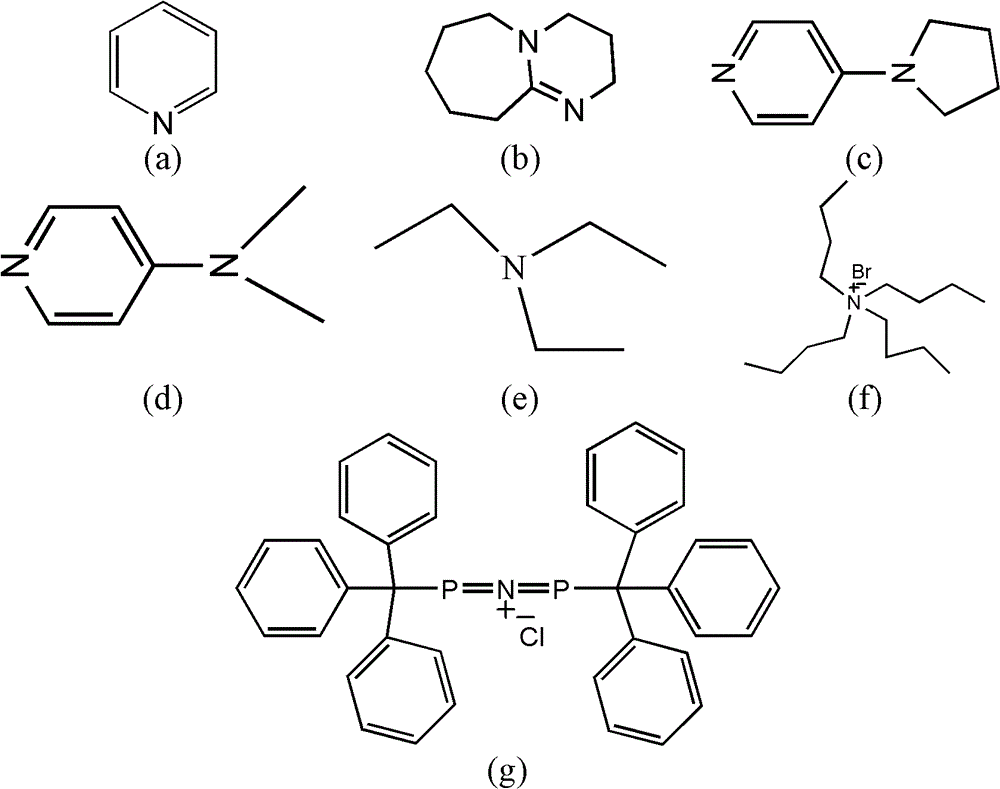
[0033]A catalyst composed of a certain amount of Cr (Salen) and pyridine (the molar ratio of the two components is 1) is placed in the reactor, and alkylene oxide is added in an amount 1000 times the molar amount of Cr (Salen). Another reactant, trimethylsilazide, was added in an amount 500 times the molar amount of Cr (Salen). After continuing to stir and react at room temperature for 4 hours, the catalyst was separated by column chromatography, and the product was analyzed by gas chromatography to obtain a conversion rate of >49% (during the resolution reaction, the complete conversion rate of resolution was 50%). The ee value of the resolved product 1-azido-2-trialkylsiloxane was 96%.
Embodiment 2
[0035] The experimental process of Example 1 was adopted, except that the molar ratio of pyridine to Cr(Salen) was 0.5, and the reaction time was 6 h. A conversion of >49% was obtained. The ee value of the resolved product 1-azido-2-trialkylsiloxane can reach 96%.
Embodiment 3
[0037] The experimental process of Example 1 was adopted, except that the molar ratio of pyridine to Cr(Salen) was 2, and the reaction time was 24 hours. A conversion of 45% was obtained. The ee value of the resolved product 1-azido-2-trialkylsiloxane can reach 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com