Chiral triptycene polyimide film and its preparation method and application of chiral molecular separation
A technology of pterene polyimide and chiral molecules, which is applied in the field of separation of chiral molecules, and can solve problems such as the few researches on chiral thin films, the problem of polymer solubility, and the difficulty in post-processing of materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Synthesis of R-triptycene hexafluoropolyimide with R-configuration 2,6-diaminotriptycene and hexafluorodianhydride.
[0034] Dissolve R-configuration 2,6-diaminotriptycene (550mg, 1.9mmol) and hexafluorodianhydride (851mg, 1.9mmol) in N-methylpyrrolidone (10ml), under argon atmosphere, 25°C Reaction 24h. Pyridine (1.5ml) and acetic anhydride (2.5ml) were added, and the reaction was continued for 1h. Then the temperature was raised to 110°C, and the reaction was carried out for 24 hours. The reaction was terminated, and the reaction solution was cooled and then poured into methanol (300ml) for precipitation, and the precipitate was collected and purified by Soxhlet extraction of methanol solution to obtain pure R-triptycene hexafluoropolyimide.
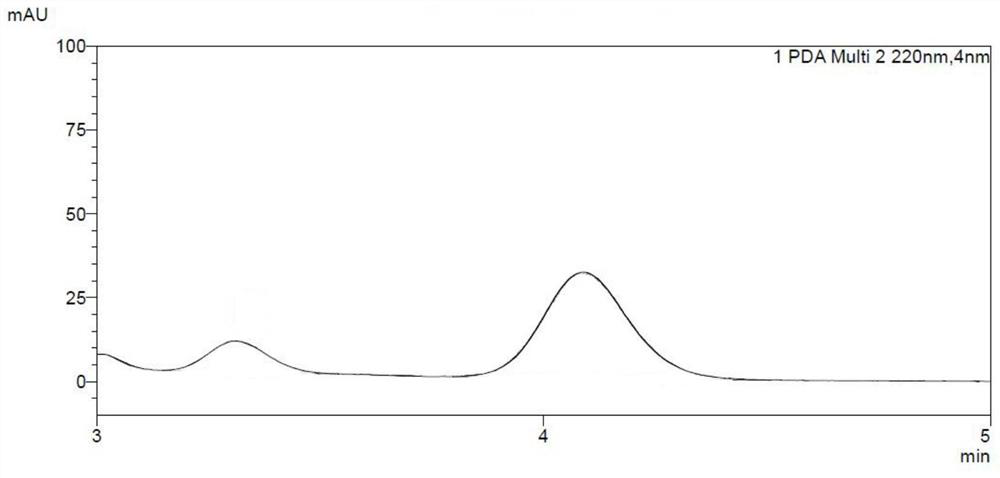
[0035] R-triptycene hexafluoropolyimide was dissolved in tetrahydrofuran and detected by gel permeation chromatography (GPC), and its degree of polymerization n was 38.
Embodiment 2
[0036] Example 2: S-configuration 2,6-diaminotriptycene and hexafluorodianhydride to synthesize S-triptycene hexafluoropolyimide.
[0037] Dissolve S-configuration 2,6-diaminotriptycene (550mg, 1.9mmol) and hexafluorodianhydride (851mg, 1.9mmol) in N-methylpyrrolidone (10ml), under argon atmosphere, 25°C React for 24 hours. Pyridine (1.5ml) and acetic anhydride (2.5ml) were added and the reaction was continued for 1 hour. Then the temperature was raised to 110° C., and the reaction was carried out for 24 hours. Terminate the reaction, and pour the reaction solution into methanol (300ml) for precipitation after it is cooled, collect the precipitate and carry out the Soxhlet extraction step of methanol solution for purification to obtain pure S-triptycene hexafluoropolyimide.
[0038] S-triptycene hexafluoropolyimide was dissolved in tetrahydrofuran and detected by gel permeation chromatography (GPC), and its degree of polymerization n was 66.
Embodiment 3
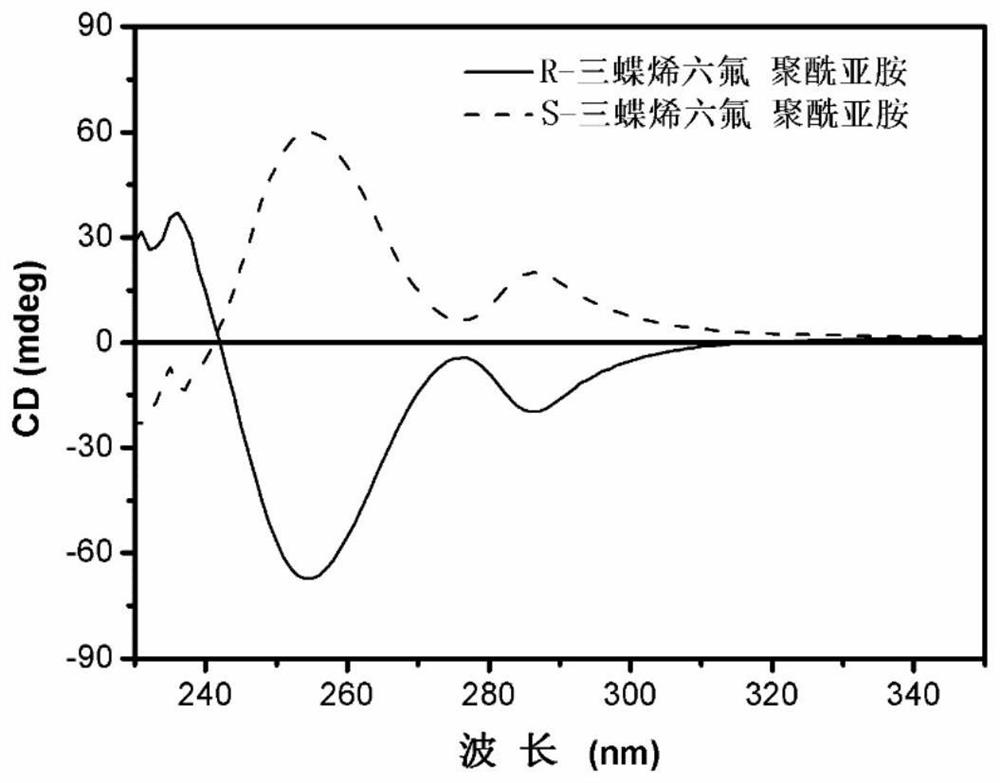
[0039] Example 3: Circular Dichroism Detection of R-triptycene hexafluoropolyimide and S-triptycene hexafluoropolyimide
[0040] R-triptycene hexafluoropolyimide and S-triptycene hexafluoropolyimide were respectively prepared into 0.2mg / ml tetrahydrofuran solutions, and then added into a detection cell with a thickness of 1mm for testing. as attached figure 1 As shown, the obtained two circular dichroism chromatograms have good symmetry and obvious peak shape, which indicates that the polymer structure contains a chiral structure, and chiral triptycene has been embedded in the polymer structure.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com