The synthetic method of ethyl demethylthiaxamate
A technique for the synthesis of ethyl demethylaminothiaxamate and its synthetic method, which is applied in the field of preparation of antibiotic drug intermediates, can solve the problems of increased impurities in cyclization products, poor product color, and difficulties in separation and purification, and achieves high industrial application value and is easy to use. Effect of large-scale production and improvement of reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
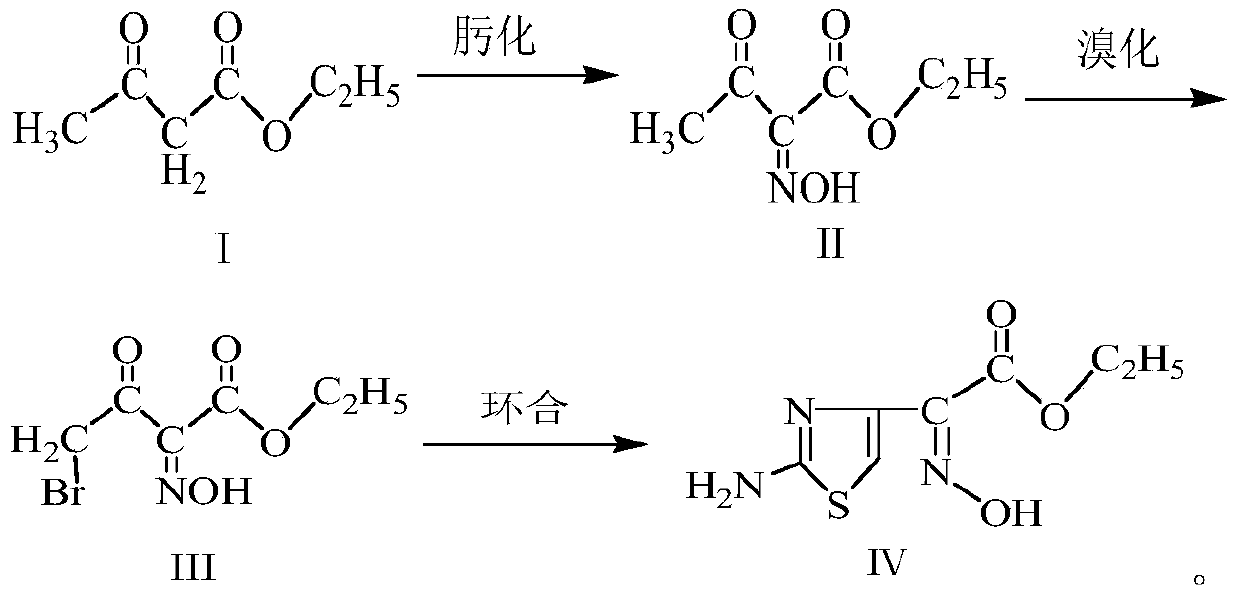
[0031] (1) Oximation reaction: Preparation of ethyl 2-hydroxyiminoacetoacetate (Ⅱ)
[0032] Add 52.5g of sodium nitrite and 450g of purified water into the three-neck flask, stir for 25min, after fully dissolving, cool down to 10-15°C, add 90g of ethyl acetoacetate, then slowly drop 74.5g of 50% concentrated sulfuric acid solution to control the reaction The temperature of the system is 10-15° C., the time for the dropwise addition is 4 hours, and the reaction is continued for 3 hours after the dropwise addition is completed. Stand still for 15min, layered, add 65g chloroform to the aqueous phase, stir for 15min, stand still for 15min, layered, add 45g chloroform again for the aqueous phase, stir for 15min, stand for 15min, layered, combine the organic phases, and distill under reduced pressure, The obtained reaction solution was used for bromination reaction.
[0033] (2) Bromination reaction: Preparation of ethyl 4-bromo-2-hydroxyiminoacetoacetate (Ⅲ)
[0034] At 30-40°C, ...
Embodiment 2
[0038] (1) Oximation reaction: Preparation of ethyl 2-hydroxyiminoacetoacetate (Ⅱ)
[0039] Add 52.5g of sodium nitrite and 435g of purified water into the three-necked flask, stir for 30min, after fully dissolving, cool down to 10-15°C, add 90g of ethyl acetoacetate, then slowly dropwise add 74.5g of 50% concentrated sulfuric acid solution to control the reaction The temperature of the system is 10-15° C., the dropwise addition time is 3 hours, and the reaction is continued for 5 hours after the dropwise addition is completed. Stand still for 15min, layered, add 55g of chloroform to the water phase, stir for 15min, stand for 15min, layered, add 55g of chloroform to the water phase, stir for 15min, stand for 15min, layered, combine the organic phases, and distill under reduced pressure, The obtained reaction solution was used for bromination reaction.
[0040] (2) Bromination reaction: Preparation of ethyl 4-bromo-2-hydroxyiminoacetoacetate (Ⅲ)
[0041] At 30-40°C, slowly ad...
Embodiment 3
[0045] (1) Oximation reaction: Preparation of ethyl 2-hydroxyiminoacetoacetate (Ⅱ)
[0046] Add 52.5g of sodium nitrite and 465g of purified water into the three-neck flask, stir for 20min, after fully dissolving, cool down to 10-15°C, add 90g of ethyl acetoacetate, then slowly add 74.5g of 50% concentrated sulfuric acid solution dropwise to control the reaction The temperature of the system is 10-15° C., the dropwise addition time is 5 hours, and the reaction is continued for 3 hours after the dropwise addition is completed. Stand still for 15min, layered, add 60g chloroform to the aqueous phase, stir for 15min, stand still for 15min, layered, add 50g chloroform for the aqueous phase, stir for 15min, stand for 15min, layered, combine the organic phases, and distill under reduced pressure, The obtained reaction solution was used for bromination reaction.
[0047] (2) Bromination reaction: Preparation of ethyl 4-bromo-2-hydroxyiminoacetoacetate (Ⅲ)
[0048] At 30-40°C, slowly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com