a g5-mos 2 Preparation method of /bcl-2 siRNA complex
A technology of bcl-2siRNA and g5-mos2, which is applied in the field of preparation of G5-MoS2/Bcl-2siRNA complex, achieves the effects of easy operation, easy synthesis and purification, good dispersion and biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] (1) Weigh 100mg (NH 4 ) 2 MoS 4 The powder was dissolved in 20 mL of deionized water, stirred magnetically for 20 min, and then ultrasonically oscillated for 10 min until the powder was completely dissolved in water. While stirring, add 0.454mL of (N 2 h 4 )·H 2 O, ultrasonic vibration for 30min. The resulting mixture was poured into a 50mL polytetrafluoroethylene-lined reactor, then heated to 200°C for 10 hours, and the reactor was taken out to cool naturally to room temperature to obtain a black solution. Centrifuge the above black solution at 10,000 rpm for 5 minutes to collect the product, which is the obtained MoS 2 . MoS was washed with water and centrifuged 2 Purification was carried out, and after repeated 10 times, the purified MoS 2 Dissolve in 10mL deionized water and store at 4°C for later use.
[0055] (2) 3.996mg LA was dissolved in 5mL DMSO, then 51.81mg EDC·HCl and 33.18mg NHS were weighed, dissolved in 2mL DMSO solution, and stirred for 3h to ...
Embodiment 2
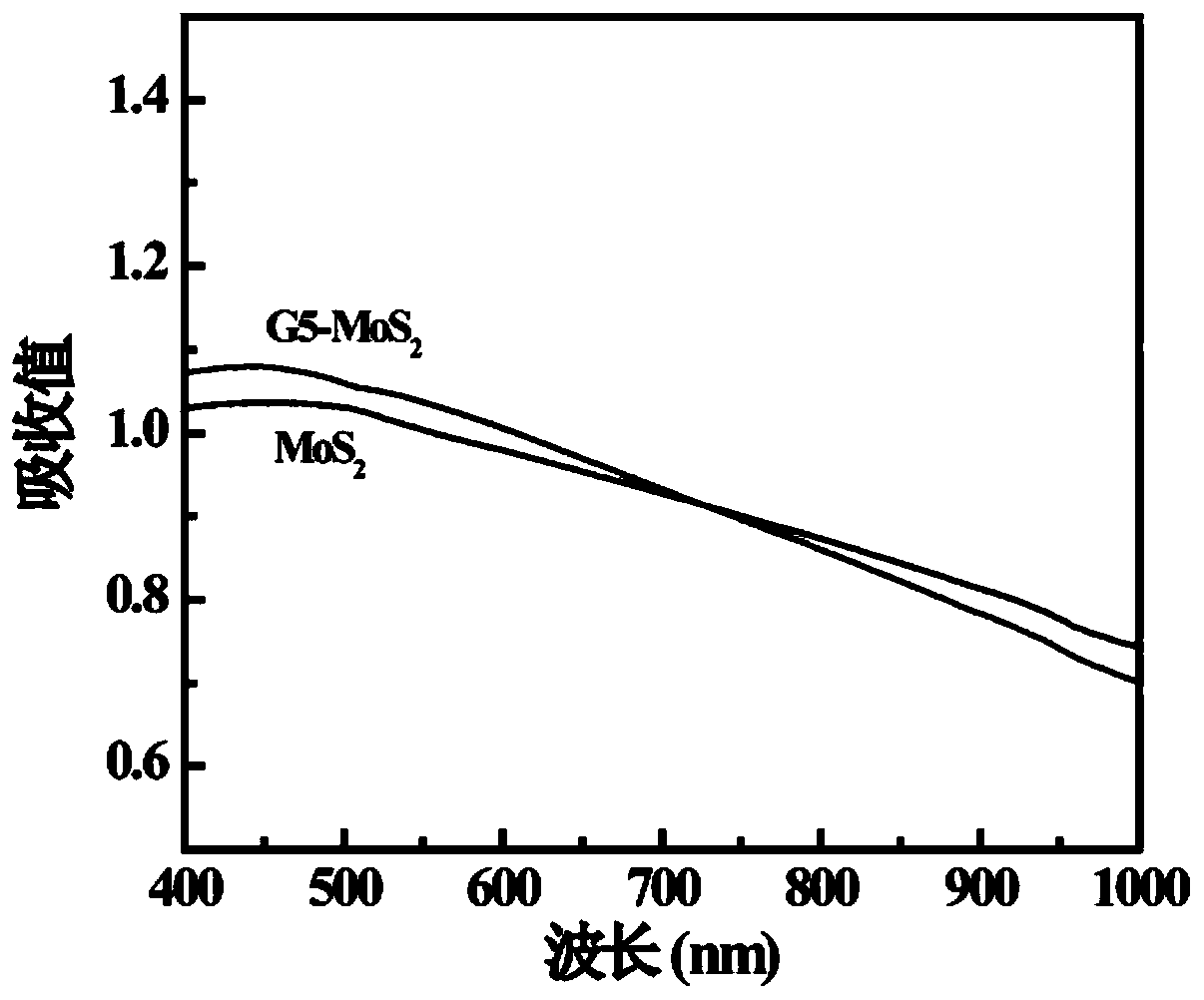
[0059] Carry out NMR characterization to the G5-LA prepared in embodiment 1 step (2), 1 HNMR characterization results such as figure 1 As shown, there is a proton peak at the chemical shift of 1.5-2.5ppm, which is a characteristic group proton peak in the molecular structure of LA. According to its and G5.NH 2 The integral area ratio between each G5.NH can be calculated 2 11.4 LA molecules were attached to it. The UV-vis results are shown in Figure 2, it can be seen that the modified G5.NH2 Before and after, the absorption characteristics of the ultraviolet absorption peak did not change significantly, indicating that the G5.NH 2 modification did not change the MoS 2 light absorption properties. TEM results such as image 3 As shown, the prepared MoS 2 (3a) presents layered structure, and modified G5.NH 2 After (3b), G5-MoS 2 A polymer is formed, the shape is close to spherical, and the particle size of the formed nanoflower is about 100-200nm. FESEM results such as ...
Embodiment 3
[0061] The MoS prepared by the method of step (1) and step (3) of embodiment 1 2 and G5-MoS 2 Dilute with PBS to obtain a solution with a concentration of 0.1 mg / mL, and take 1 mL of the solution for hydrodynamic diameter (6a) and surface potential (6b) characterization by a Malvern laser particle size analyzer (Malvern, MK, 633nm laser). The result is as Figure 6 As shown, the unmodified MoS 2 With large hydrodynamic diameter and negative surface potential, it is not suitable for gene transfection. while G5-MoS 2 The particle size of G5.NH was significantly reduced, indicating that 2 The modification improves the dispersion of the material. Meanwhile, G5-MoS 2 The charge carried on the surface has also changed from negative to positive, making it easier to be phagocytized by cells.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com