Preparation method and application of CIK (Cytokine-induced Killer) modified by anti-human CD19 chimeric antigen receptor
A chimeric antigen receptor, -CD3 technology, applied in genetically modified cells, botany equipment and methods, biochemical equipment and methods, etc. Normal tissue damage and other problems, to achieve the effect of preventing recurrence, improving immunity, and enhancing cell activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
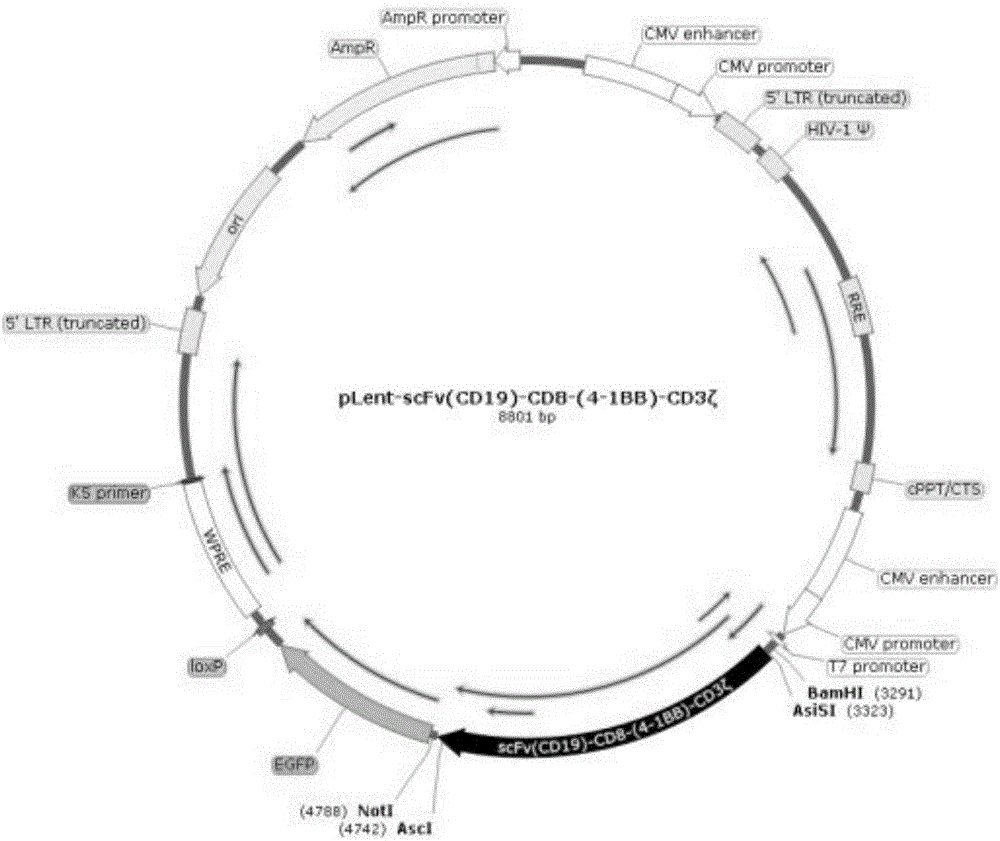
[0030] Example 1: Inserting the fusion gene fragment scFv(CD19)-CD8-(4-1BB)-CD3ζ into the lentiviral expression vector pLent-C-GFP
[0031] The fusion gene fragment scFv(CD19)-CD8-(4-1BB)-CD3ζ and the lentiviral expression vector pLent-C-GFP (purchased from Vigene) were double-digested with restriction endonucleases KpnⅠ and AsiSI, and the restriction enzyme digestion system See instructions. After the digested products were separated by agarose gel electrophoresis, DNA fragments were recovered using an agarose gel DNA fragment kit (purchased from Omega). The recovered fusion gene fragment was ligated with the linear vector by T4 ligase (purchased from TaKaRa Company) at 16° C. for 8 hours. Transfer the ligation product into E.coli Top10, pick a single clone after culturing at 37°C for 10 hours, screen the single clone inserting the fusion fragment by PCR, and extract the plasmid with a plasmid extraction kit (purchased from Omega) after culturing at 37°C for 12 hours , see ...
Embodiment 2
[0032] Example 2: Preparation of cytokine-induced killer cells modified by chimeric antigen receptor scFv(CD19)-CD8-(4-1BB)-CD3ζ
[0033] (1) Packaging preparation of lentivirus
[0034] Anti-CD19CAR opinion Figure 9 ;
[0035] The sequence of each module of Anti-CD19:
[0036] 1) CAR guide sequence (Leader)
[0037] 2) Humanized anti-human B-lymphoma CD19 single-chain antibody (VL-Linker-VH)
[0038] 3) CD8Hinge area (CD8Hinge)
[0039] 4) Transmembrane region of CD8 (CD8Tm)
[0040] 5) Intracellular domain of 4-1BB (4-1BB lc)
[0041] 6) Intracellular domain of CD3ζ (CD3lc)
[0042] (2) Preparation of cytokine-induced killer cells (CIK)
[0043] Take 50ml of the patient's autologous peripheral blood, and use TBD sample density separation medium (purchased from Tianjin Haoyanghuake Biology Co., Ltd.) to centrifuge at 900g for 25 minutes to separate peripheral blood mononuclear cells. After inducing culture for 24 hours with a culture medium (purchased from CORNING C...
Embodiment 3
[0053] Example 3: Anti-tumor effect of chimeric antigen receptor scFv(CD19)-CD8-(4-1BB)-CD3ζ modified CIK
[0054] (1) Related work before treatment:
[0055] Before the patient is treated with chimeric antigen receptor-modified T cells, it is necessary to confirm whether the patient's tumor antigen expresses the tumor antigen targeted by the chimeric antigen receptor-modified T cells.
[0056] Before receiving chimeric antigen receptor T cell therapy, patients must undergo a general physical examination, especially heart, lung, liver, kidney function and blood tests, to ensure the safety of the patient's treatment. The specific examination is as follows:
[0057] 1: Cardiac function test:
[0058] Before the treatment, the heart function of the patient is graded. If the heart function of the patient is grade 3 or above, the patient is not suitable for this treatment.
[0059] 2: Pulmonary function test:
[0060] Pulmonary function tests usually include pulmonary ventilatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com