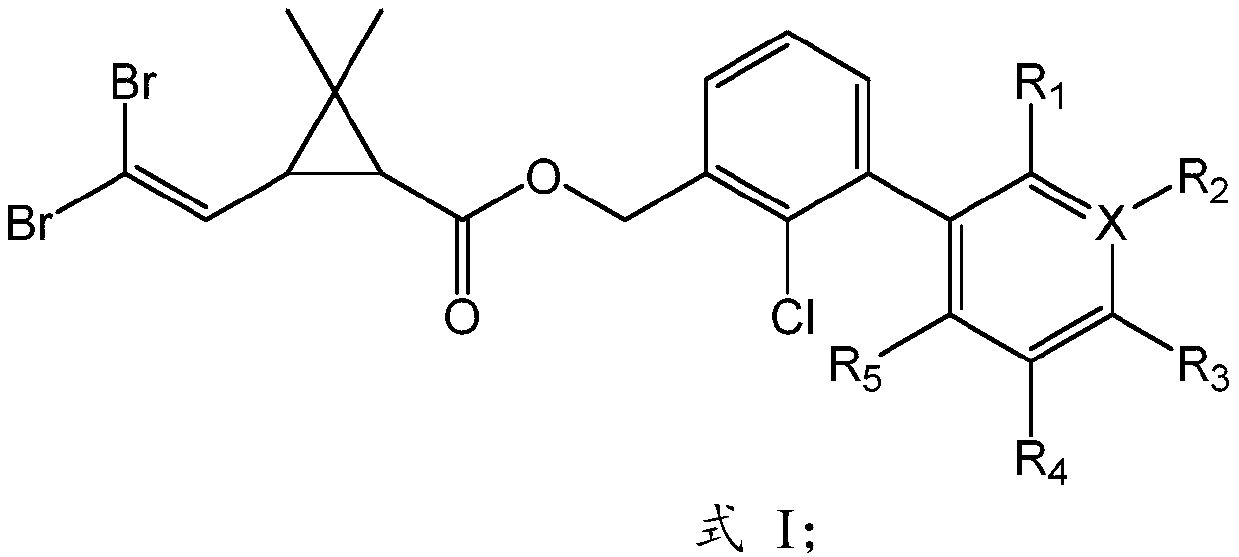
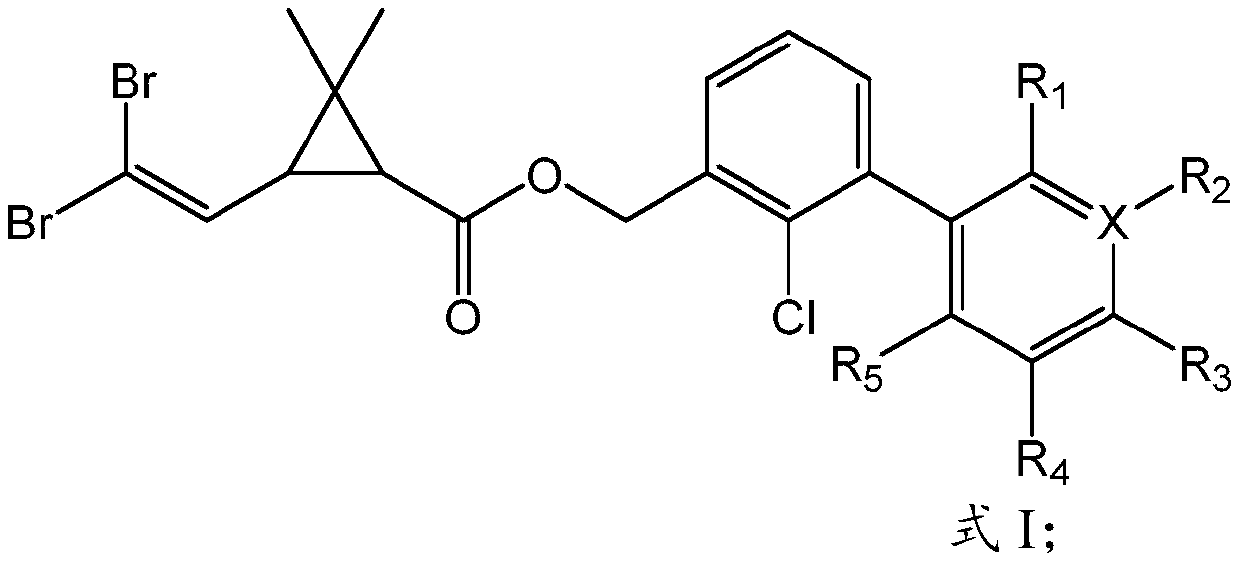
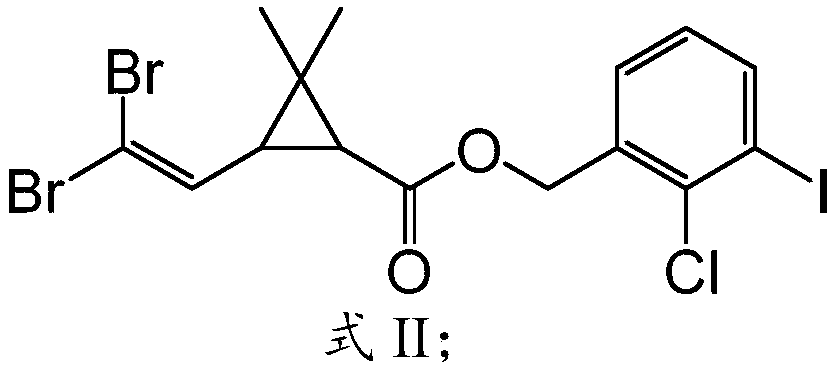
Novel chlorine-containing bifenthrin and preparation method and application thereof
A new type of technology for bifenthrin, which is applied in the field of preparation of new bifenthrin-containing bifenthrin, can solve the problems of poor drug efficacy, achieve strong biological activity, simple preparation method, and good killing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of novel bifenthrin (SZ-B-02):
[0037]
[0038] First, obtain Intermediate A through continuous reaction, add 548.0mg (1.0mmol) Intermediate A in a 100ml round bottom flask, add 850.0mg (4.0mmol) of potassium phosphate simultaneously, add 36.6mg (0.05mmol) of [ 1,1-bis(diphenylphosphino)ferrocene]palladium dichloride, then add 236.1mg (1.5mmol) of 2-chloro-5-pyridineboronic acid, dissolve in 30ml of toluene solution, and place in an oil pan React at 100°C for 12 hours; secondly, determine the end point of the reaction by thin layer chromatography, filter the above reaction solution with suction to remove the solid in the reaction, and rotary evaporate the filtrate after suction filtration to obtain the crude product; finally, extract and wash , drying, filtration, rotary evaporation and column chromatography and other purification processes to obtain the target compound SZ-B-02 with a yield of 71%. 1 H NMR (400MHz, CDCl 3 )δ8.43(d,1H),7.75(dd,J=8.2,2.3...
Embodiment 2
[0040] Preparation of novel bifenthrin (SZ-B-04):
[0041]
[0042] First, obtain Intermediate A through continuous reaction, add 548.0mg (1.0mmol) Intermediate A in a 100ml round bottom flask, add 850.0mg (4.0mmol) of potassium phosphate simultaneously, add 36.6mg (0.05mmol) of [ 1,1-bis(diphenylphosphino)ferrocene]palladium dichloride, then add 263.8mg (1.5mmol) of 3,4,5-trifluorophenylboronic acid, dissolve in 30ml of toluene solution, in the oil domain React in the pot at 100°C for 12 hours; secondly, determine the end point of the reaction by thin layer chromatography, filter the above reaction solution with suction to remove the solid in the reaction, and rotary evaporate the filtrate after suction filtration to obtain the crude product; finally, extract , washing, drying, filtration, rotary evaporation and column chromatography to obtain the target compound SZ-B-04 with a yield of 65%. 1 H NMR (400MHz, CDCl 3 )δ7.46(dd, J=7.7,1.5Hz,1H),7.36(t,J=7.6Hz,1H),7.26(dd,1H...
Embodiment 3
[0044]Preparation of novel bifenthrin (SZ-B-05):
[0045]
[0046] First, obtain Intermediate A through continuous reaction, add 548.0mg (1.0mmol) Intermediate A in a 100ml round bottom flask, add 850.0mg (4.0mmol) of potassium phosphate simultaneously, add 36.6mg (0.05mmol) of [ 1,1-bis(diphenylphosphino)ferrocene]palladium dichloride, then add 284.8mg (1.5mmol) of 4-trifluoromethylbenzeneboronic acid, dissolve in 30ml of toluene solution, and place in an oil pan React at 100°C for 12 hours; secondly, determine the end point of the reaction by thin layer chromatography, filter the above reaction solution with suction to remove the solid in the reaction, and rotary evaporate the filtrate after suction filtration to obtain the crude product; finally, extract and wash , drying, filtration, rotary evaporation and column chromatography and other purification processes to obtain the target compound SZ-B-05 with a yield of 39%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com