Retinal progenitor cell and preparation thereof having function of treating degenerative retinal diseases
A technology for retinal progenitor cells and retinal degeneration, applied in the field of stem cells, can solve the problems of unresolved foreign vectors, abnormal virus integration and expression, and unsuitable for clinical applications.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
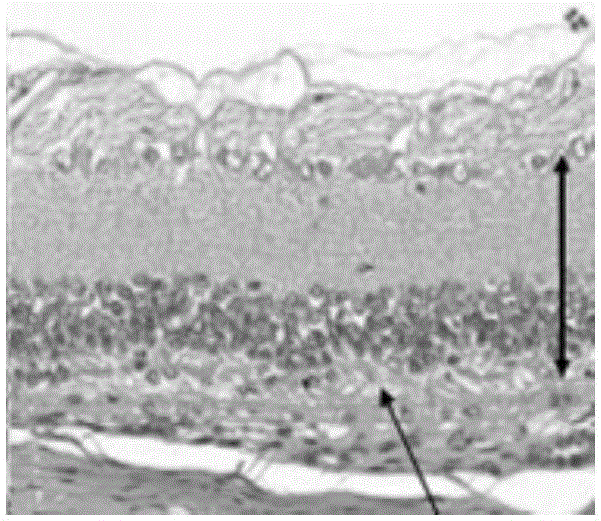
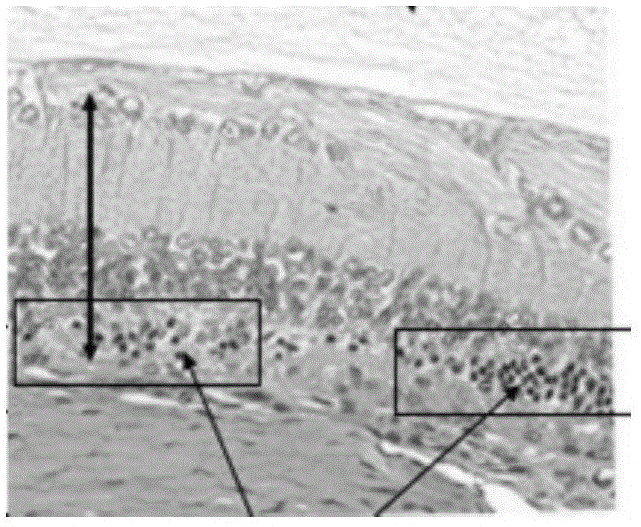
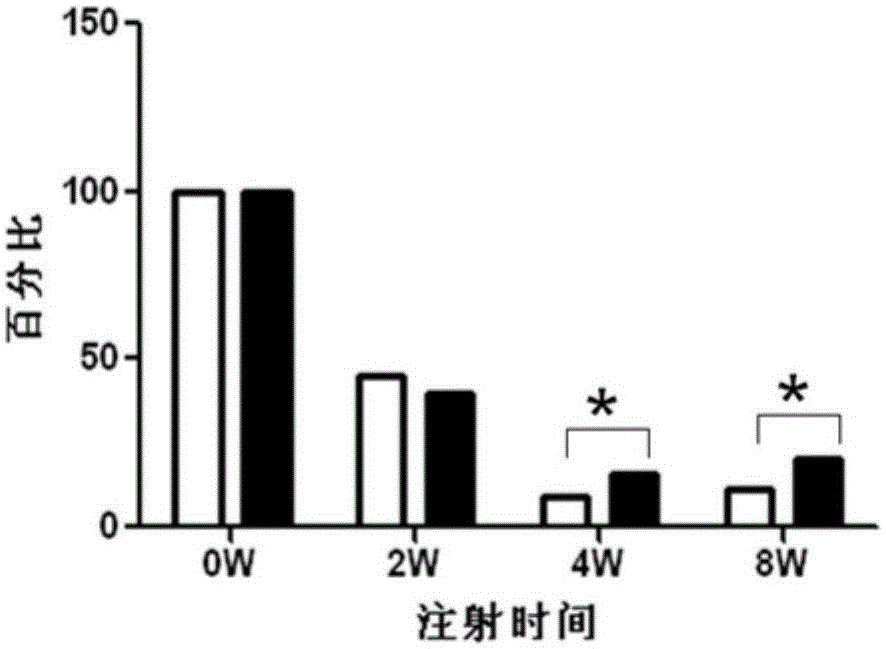
Image
Examples
Embodiment 1
[0063] Example 1 The cultivation of retinal progenitor cells
[0064] Sphere-forming culture of retinal progenitor cells: After the tissue is sterilized, take the retinal tissue, digest it with enzymes, centrifuge at 1000r for 3 minutes, take the precipitate, and use DMEM / F12 basal medium (add N-2 supplement, the concentration is 1:100, Epidermal growth factor (EGF, concentration 10ng / ml, basic growth factor bFGF, concentration 10ng / ml, L-glutamine, concentration 1:100) mix and count according to 1-2×10 5 / cm 2 Inoculate in low-adsorption T25 culture flasks, place at 37°C, 5% CO 2 Cultured in a constant temperature incubator, the next day the cells formed into spheres.
[0065]Preparation of sodium alginate gel: Use deionized water as the solvent of sodium alginate, first weigh the sodium alginate powder, slowly add the powder into a centrifuge tube containing a small amount of solvent (20ml), and add the powder while adding For the remaining solvent, shake it with a vortex...
Embodiment 2
[0068] Example 2 Identification of Retinal Progenitor Cells
[0069] The vigorously proliferating fifth-generation retinal progenitor cells cultured in Example 1 were identified by flow cytometry: using TrypLE TM -Express digested retinal progenitor cells, resuspended cells in DPBS and counted according to 0.5×10 5 The amount of cells was evenly distributed to the flow detection tubes, and then the identification antibody (Nestin / Pax6 / / Ki67 / Chx10 / Sox2 / CD38 / HLA-DR) and the isotype control antibody were incubated at room temperature in the dark for 15min, and then added Centrifuge 1ml DMEM / F12 at 500-800rpm for 3min. Resuspended with 30μL DPBS for detection on the machine.
[0070] Identification antibodies and isotype control antibodies were purchased from BD and Abcam.
[0071] The identification criteria are: the expression of Nestin is greater than 90%; the expression of Pax6 is greater than 70%; the expression of Ki-67 is greater than 60%; the expression of Chx10 is grea...
Embodiment 3
[0074] Example 3 Preparation of Retinal Progenitor Cell Preparation
[0075] The retinal progenitor cells with the preservation number CGMCC NO.10419 were suspended in HBSS balanced salt solution, and the cell concentration was 4×10 7 individual / mL.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com