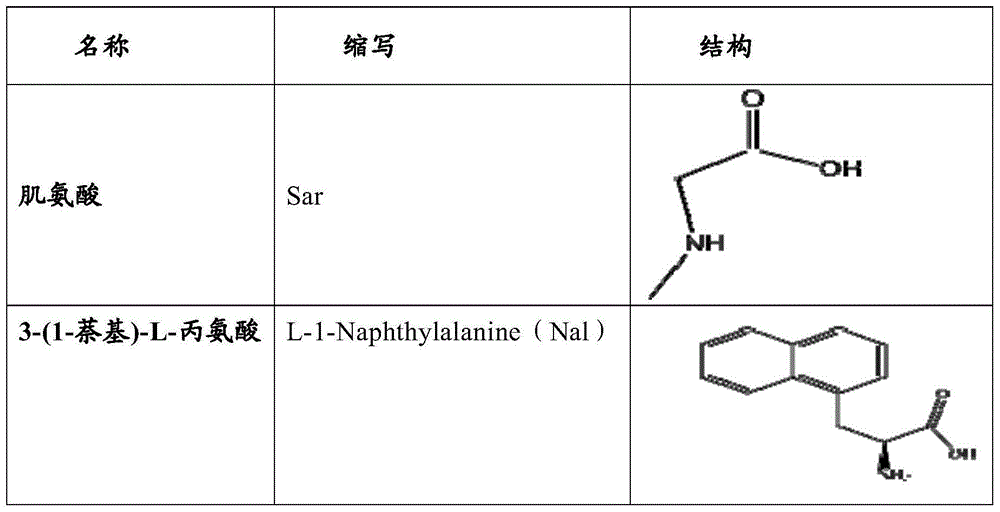
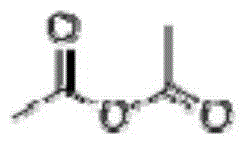Erythropoietin mimetic peptide, and preparation method and application thereof
An erythropoietin and peptide-mimicking technology, applied in the field of biomedicine, can solve the problems of low EC50, limit the clinical application of recombinant human EPO, reduce the efficacy of drugs, etc., and achieve the effect of prolonging the half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Synthesis of SEQ ID NO:1
[0037] The direction of solid-phase synthesis is from C-terminal to N-terminal, starting with the C-terminal lysine of the peptide shown in SEQ ID NO: 1, extending the first amino acid of the sequence (glycine or acetylated glycine), and then simultaneously or The ε-amino protecting group of the linker lysine is successively removed to continue the synthesis of the second branched chain.
[0038] The steps are as follows: soak the resin, remove the amino protecting group of the resin, wash and monitor
[0039] The removal of α-amino protecting group and resin amino protecting group Fmoc is selected from piperidine (PIP), the concentration is 20-25% (PIP: DMF), and the time is 20-50min. The solvent used in the synthesis process is selected from dimethylformamide (DMF) or dichloromethane (DCM), the coupling reagent is selected from a carbodiimide type reagent or a benzotriazolium salt type reagent, and 1-hydroxybenzotriazole (HOBt) o...
Embodiment 2
[0040] Example 2 Increased production of endogenous erythropoietin in vitro
[0041] The sequence selected in the present invention is: SEQ ID NO: 1, and the zinc ion concentration is 0.0001, 0.01, 0.1, 1, 10, 100 mM.
[0042] Human cells derived from liver cancer (Hep3B) were inoculated into 35mm culture dishes and kept at 37°C, 20% O 2 , 5%CO 2 In minimum essential medium (MEM),
[0043] Growth was in Earle's balanced salt solution (Mediatech Inc., Herndon VA), 2 mM L-glutamine, 0.1 mM non-essential amino acids, 1 mM sodium pyruvate, and 1% FBS. When the cell layer was confluent, the original medium was replaced with OPTI-MEM medium (Invitrogen Life Technologies, Carlsbad CA), and then incubated at 37°C, 20% O 2 , 5%CO 2 Incubate the cell layer for about 24 hours under the above conditions. Then, the compound of the present invention mixed with different concentrations of zinc ions or different concentrations of zinc ions (negative control) was added to the current cu...
Embodiment 3
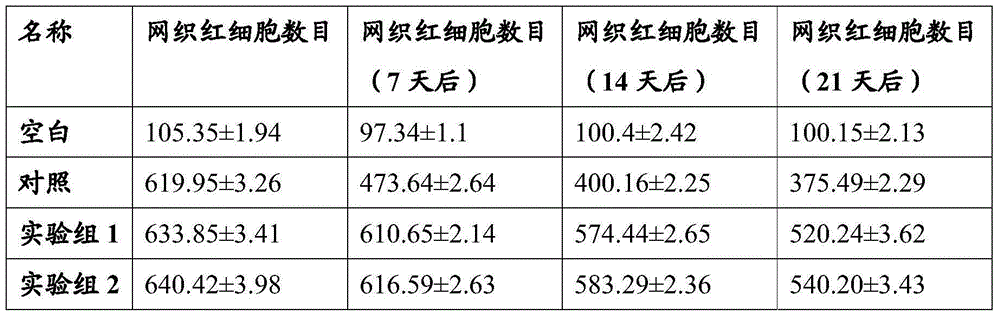
[0044] Example 3 Effects of erythropoietin mimetic peptide on mice
[0045] Mice were used to evaluate and compare the effects of erythropoietin mimetic peptide and erythropoietin protein on mouse erythropoiesis under different zinc ion concentrations.
[0046] The sequence selected in the present invention is: SEQ ID NO: 1, and the concentration of zinc ions is 0.0001, 0.001, 0.01, 0.1, 1, 10, 100 mM of zinc ions per milligram of polypeptide.
[0047] Among them, EPO drugs were purchased from Shenyang Sansheng Pharmaceutical Co., Ltd.;
[0048] Kunming mice were purchased from the Shanghai Experimental Animal Center of the Chinese Academy of Sciences, weighing 20-30 g, all male mice, and the number of animals in each group in the experiment: 10, divided into 10 groups.
[0049] Among them, the mice in the positive control group were injected with erythropoietin protein, and the mice in the negative control group were blank controls. The mice were subcutaneously injected w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com