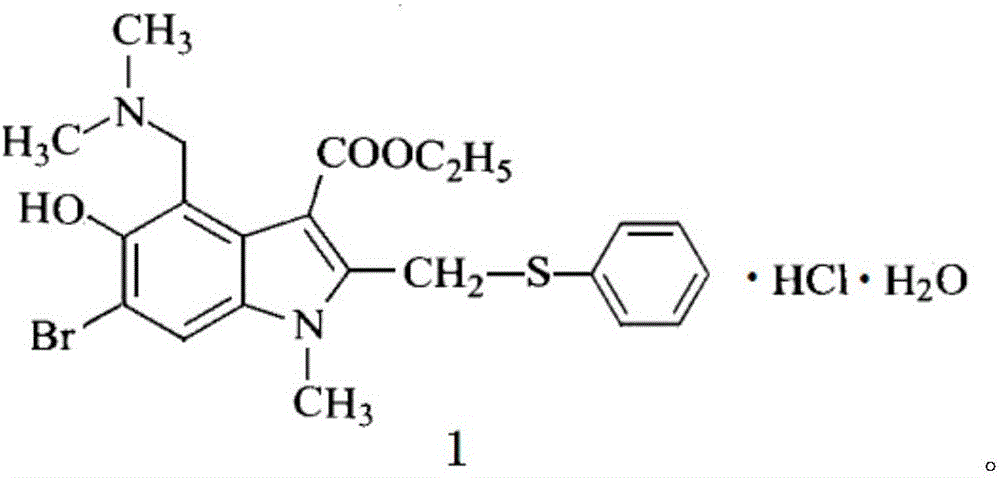
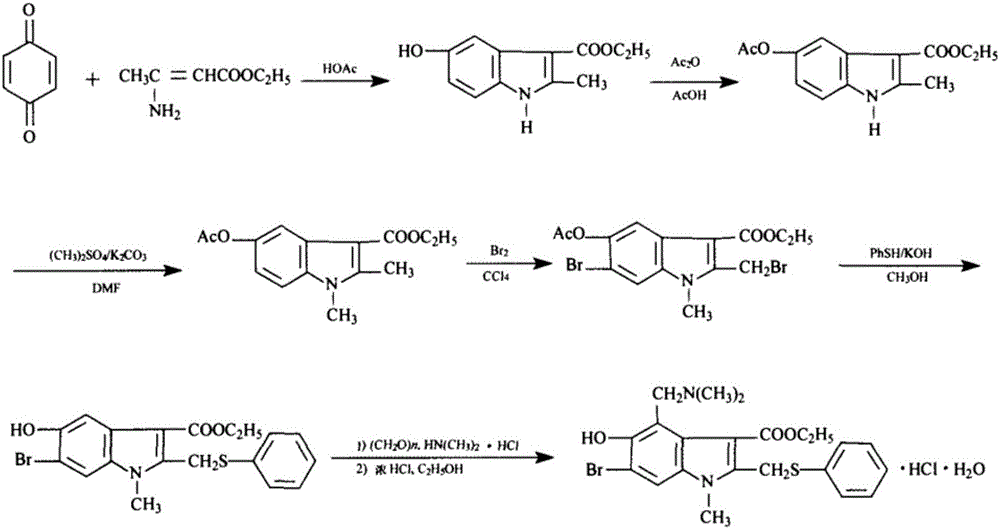
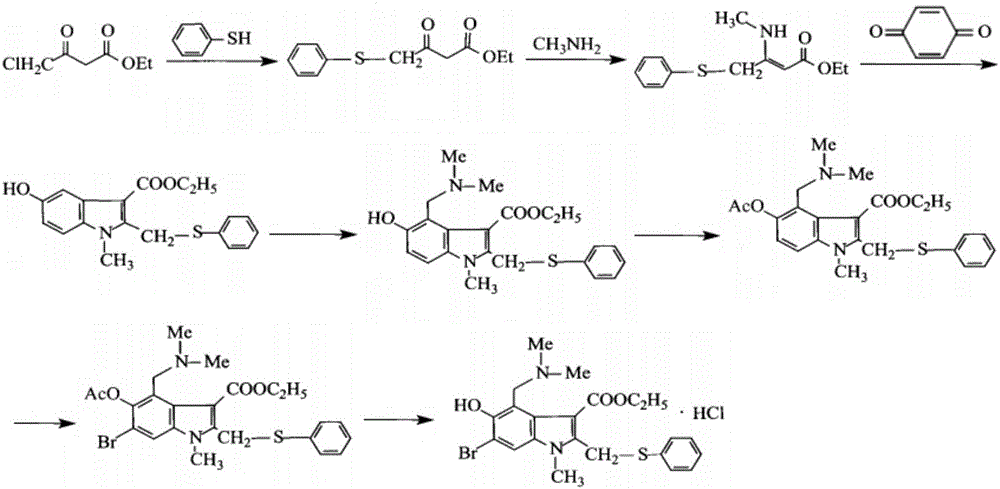
Preparation method of arbidol hydrochloride
A technology of arbidol hydrochloride and molar ratio, which is applied in the field of medicine and chemical industry, can solve the problems of low total reaction yield, low total yield and the like, and avoid the problems of low reaction yield, low production cost and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0039] Example 1-1: Preparation of 4-acetoxyaniline (7)
[0040] P-nitrophenol (9, 1.0mol, 139.1g) was added to 1000mL of acetone, 151.8g (1.5mol) of triethylamine was added, 196.3g of acetyl chloride (2.5mol) was added dropwise at room temperature, the dropwise was completed within 1 hour, and reflux reaction 1 Hours, naturally cooled to room temperature, the reaction solution was poured into 2000g of ice water, stirred, filtered, the filter cake was washed with water, and vacuum-dried to obtain 183.2g of p-nitrophenyl acetate (8) crude product. The next reaction was carried out directly without further purification.
[0041] Add 3000mL of water and 3000mL of ethanol to the reaction flask, add reduced iron powder (139.6g, 2.5mol) and ammonium chloride (133.7g, 2.5mol) under stirring, heat to reflux for 1h, and add the above-prepared p- Nitrophenyl acetate (8, 183.2g), continued to reflux for 2h, and TLC confirmed that the reaction was complete. Stop the reaction, suction fi...
Embodiment 1-2
[0042] Embodiment 1-2: Preparation of 4-acetoxyaniline (7)
[0043] P-nitrophenol (9, 1.0mol, 139.1g) was added to 1000mL of acetone, 158.2g (2mol) of pyridine was added, 196.3g of acetyl chloride (2.5mol) was added dropwise at room temperature, the dripping was completed within 1 hour, and the reaction was refluxed for 3 hours, naturally After cooling to room temperature, the reaction solution was poured into 2000 g of ice water, stirred, filtered, the filter cake was washed with water, and vacuum-dried to obtain 187.5 g of crude p-nitrophenyl acetate (8). The next reaction was carried out directly without further purification.
[0044] Add 3000mL of water and 3000mL of ethanol to the reaction flask, add reduced iron powder (195.5g, 3.5mol) and ammonium chloride (187.2g, 3.5mol) under stirring, heat to reflux for 1h, and add in batches the obtained For p-nitrophenyl acetate (8), the reaction was confirmed to be complete by TLC after continuing to reflux for 2 h. Stop the re...
Embodiment 2-1
[0045] Example 2-1: Preparation of ethyl 5-acetoxy-2-methylindole-3-carboxylate (5)
[0046] Add 2000mL of dichloromethane into the reaction flask, add 4-acetoxyaniline (7, 120.9g, 0.8mol) under stirring, heat to dissolve, add 104.1g of ethyl acetoacetate (6, 0.8mol) and catalyst bromine Indium InBr 3 (8, 2.8g, 0.008mol), after reflux reaction for 2h, TLC confirmed that the reaction was complete. Stop the reaction, suction filter while it is hot, and remove the solvent by rotating the filtrate under reduced pressure to obtain 203.7 g of the enaminone intermediate; the yield is 96.7%. The next reaction was carried out directly without further purification.
[0047] Add 203.7 g of the above-mentioned enaminone intermediate into a reaction flask containing 2000 mL of DMF, and add K respectively under stirring. 2 CO 3 (345.5g, 2.5mol), catalyst Pd(OAc) 2 (9g, 0.04mol), and oxidant Cu(OAc) 2 (480g, 2.4mol), heated to 80°C for 3 hours, TLC confirmed that the reaction was compl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com