Neutral endopeptidase inhibitor salt crystal form and preparation method thereof
A technology of crystal form and calcium salt, applied in the field of medicinal chemistry, can solve problems such as undisclosed preparation methods, and achieve the effects of good fluidity, high stability and small electrostatic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Preparation of Compound 1(A-3)
[0051]
[0052] Dissolve A-1 (300g) in 2400ml of absolute ethanol at room temperature; heat to 62°C, slowly add 140g of thionyl chloride dropwise and then raise the temperature to 65°C for 2.5h; distill under reduced pressure to obtain a white solid, add 1200ml of n-hexane After stirring, evaporate to dryness under reduced pressure, then add 1200ml of n-hexane and beat in ice bath for 60min; filter, rinse the solid with n-hexane, and dry at 35°C for 10h to obtain 246g of white solid (A-2).
[0053] At room temperature, add 246g of A-2 into a flask containing 2460ml of isopropyl acetate (IPAC) and 81.3g of succinic anhydride; add 92g of triethylamine at low temperature, and react at room temperature until A-2 is completely consumed; The solution was then stirred for 10 min, separated, and the organic phase was washed with 1000 ml×6 water until pH=5, and the obtained organic phase containing A-3 was used for subsequent preparation.
Embodiment 2
[0055] Preparation of compound 1 calcium salt (A-5) crystal form.
[0056]
[0057] At room temperature, drop 1.05 equivalent sodium hydroxide aqueous solution (1mol / L) into the organic phase obtained in Example 1, stir at 40°C for 2h, separate the liquids, extract the aqueous phase with 600mL×4 IPAC, and pressurize and rotary evaporate the IPAC to obtain Aqueous solution containing A-4;
[0058] After heating the aqueous solution containing A-4 to 70°C, slowly add calcium chloride aqueous solution (63g calcium chloride dissolved in 300ml water) dropwise at a uniform speed, after the dripping is completed, keep stirring at 90°C for 0.5 hours, then cool down to 50°C, filter, The filter cake was washed with 300 mL of water to obtain a white solid, which was dried under reduced pressure at 50° C. for 12 h to obtain 250 g (purity: 99.66%) of the crystal form of compound 1 calcium salt (A-5).
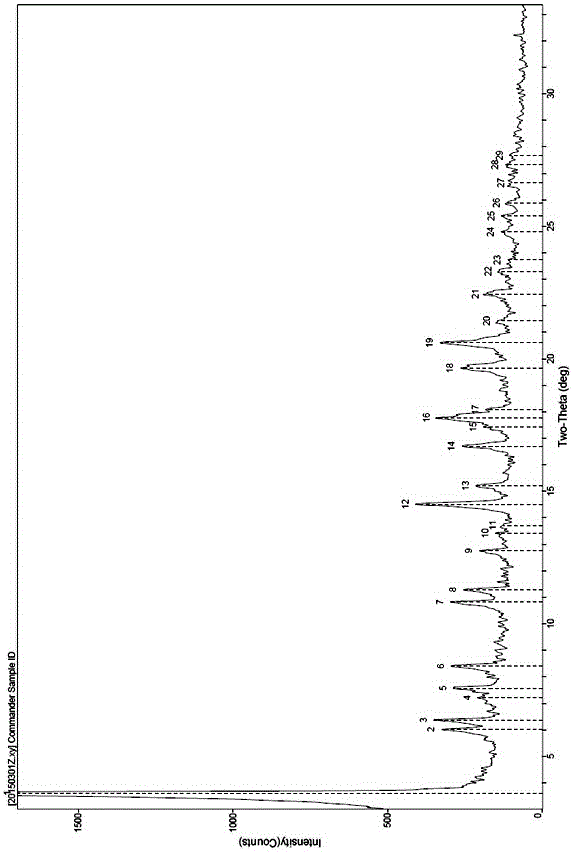
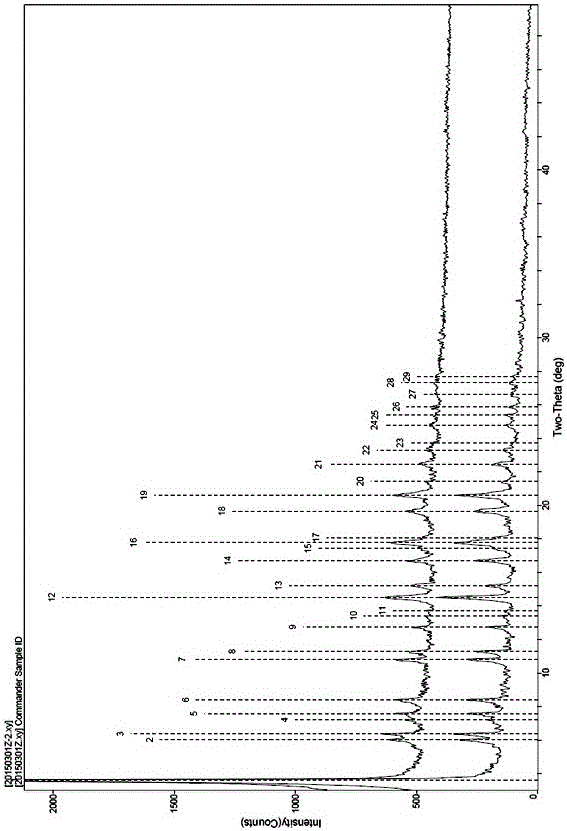
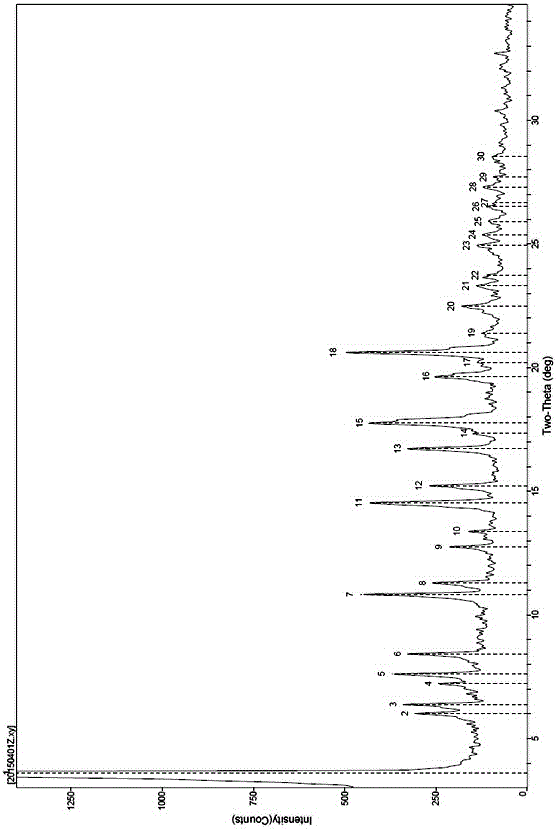
[0059] The obtained compound 1 calcium salt (A-5) sample is repeatedly detected twic...
Embodiment 3
[0063] Preparation of compound 1 calcium salt (A-5) crystal form.
[0064]
[0065] At room temperature, drop 1.0 equivalent of sodium hydroxide aqueous solution (1mol / L) into the organic phase obtained in Example 1, stir at 40°C for 2 hours, then add 0.05 equivalent of sodium hydroxide aqueous solution, stir for 1 hour, separate the liquids, and use 600 mL of the aqueous phase ×4 IPAC extraction to obtain an aqueous solution containing A-4;
[0066] After heating the aqueous solution containing A-4 to 75°C, slowly add calcium chloride aqueous solution (63g calcium chloride dissolved in 400ml water) dropwise at a uniform speed, after the dripping is completed, keep stirring at 95°C for 0.5 hours, then cool down to 50°C, filter, The obtained white solid was dried under reduced pressure at 60° C. for 8 h to obtain 230 g (purity: 99.26%) of compound 1 calcium salt (A-5) crystal form.
[0067] Gained compound 1 calcium salt (A-5) sample is repeatedly detected twice through X-r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com