Diagnostic kit for jointly detecting HIV antigen and HIV antibody and preparation method of diagnostic kit
A diagnostic kit, antigen-antibody technology, applied in the field of virus protein immunoassay, can solve the problems of rapid attenuation of detection signal value, low-end detection sensitivity, inconsistent attenuation speed, etc., and achieve the degree of detection automation without environmental pollution and coating effect. Improved, high-sensitivity effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Preparation of a diagnostic kit for combined detection of HIV antigen and antibody
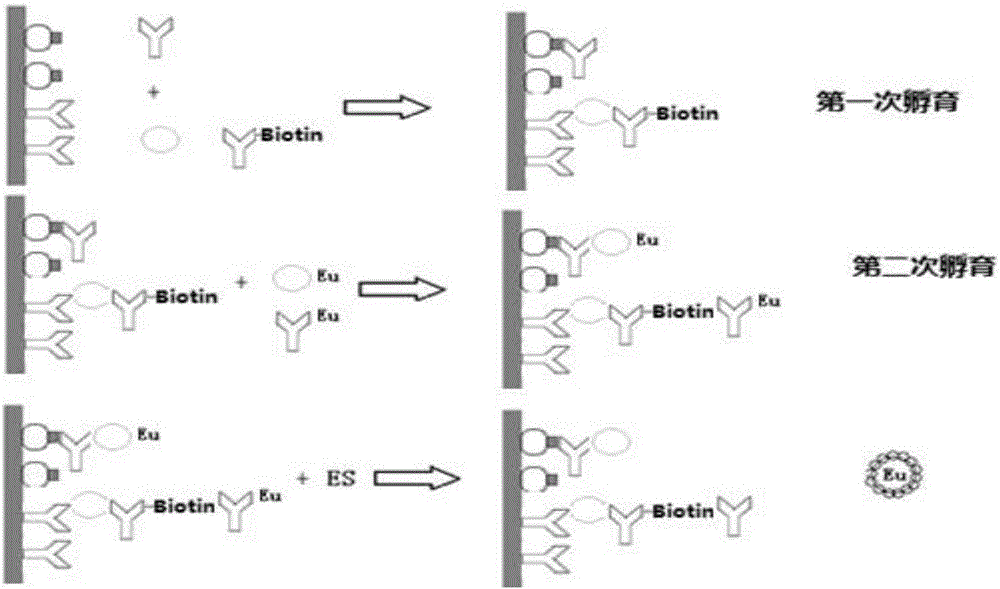
[0033] In this example, using Eu 3+ As a fluorescent marker, the specific operation is as follows:
[0034] 1. Obtaining of coated board:
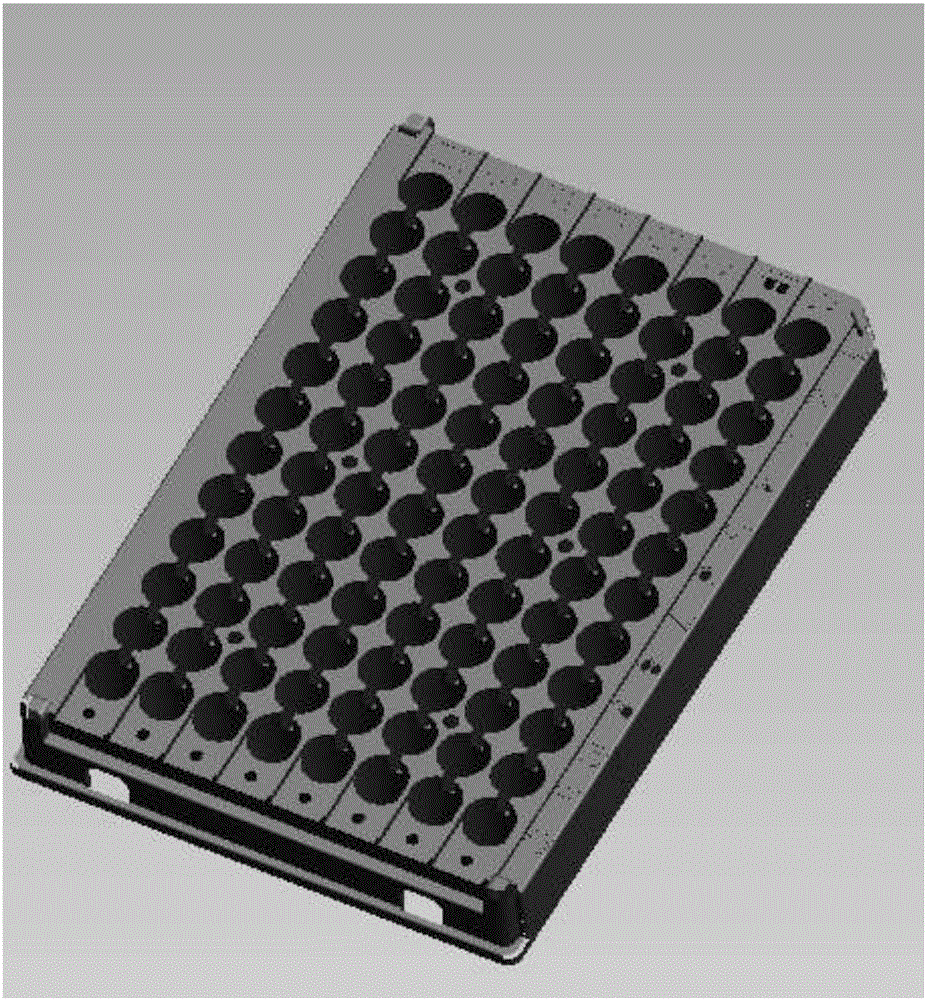
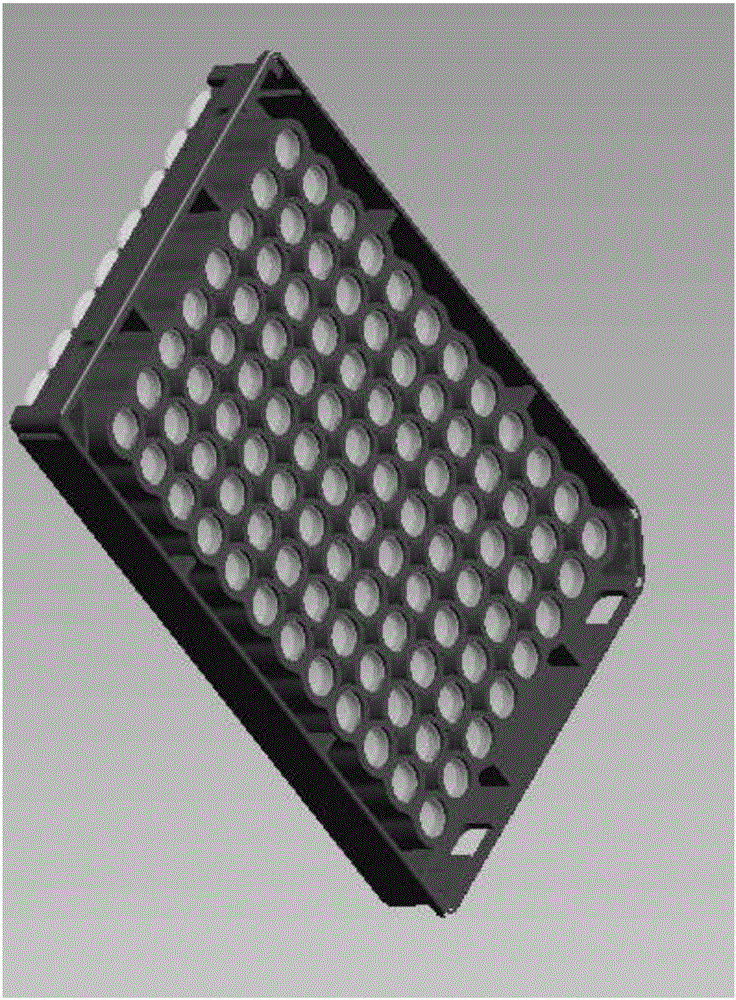
[0035] The coated reaction plate of the present invention is different from the coated reaction plate in the prior art. A special mold is used in the preparation process. The overall plate frame is made of completely opaque black polystyrene. Through-holes corresponding to the aperture of the board (such as figure 2 shown); the ELISA plate is made of materials with excellent light transmission. Before coating the antibody, it is irradiated with cobalt 60 for 30 days to obtain high adsorption. The adsorption pretreatment coated reaction plate is ready for use, as attached image 3 shown.
[0036] The model of the treatment agent is HM, purchased from Shenzhen Faipeng Biological Co., Ltd. The treatment process is as follows: mixing th...
Embodiment 2
[0043] Example 2 Detection of HIV antigens and antibodies using a combined detection kit
[0044] Each plate of the reaction plate is provided with 3 wells of negative control, 2 wells of positive control of HIV-1 antibody, 1 well of positive control of HIV-2 antibody and HIV-1p24 antigen. For samples, react at room temperature (18°C to 28°C) for 1 hour on a slow shaker;
[0045] Wash 6 times with washing solution, add 100 μL europium-labeled working solution to each well, and react for 30 minutes at room temperature (18°C-28°C) on the slow gear of the shaker;
[0046] Wash 6 times with washing solution, add 100 μL enhancement solution to each well, and react for 5 minutes at room temperature (18°C-28°C) on the slow gear of the shaker;
[0047] Place the reaction plate in the fluorescence detector and select the corresponding program to read the value.
Embodiment 3
[0048] Embodiment 3 Comparative experiment between kit of the present invention and enzyme immunodiagnostic kit
[0049] Utilize the time-resolved immunofluorescence diagnostic kit for the joint detection of HIV antigen and antibody of the present invention and the common enzyme-immune diagnostic kit (Wantai) to simultaneously detect 1025 HIV samples, if the initial test results of the samples are inconsistent, then use the two detection kits to retest Check it out.
[0050] The test results showed that the test results of 5 samples were inconsistent. Taking the detection results of the ELISA method as a reference, the sensitivity of the time-resolved immunofluorescence method reaches 99.2%, the specificity reaches 100%, and the coincidence rate reaches 99.5% (Table 1).
[0051] Table 1 Comparison of HIV sample detection results by two detection methods
[0052]
[0053] comparison of specificity
[0054] The above-mentioned 5 samples with inconsistent preliminary test r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com