Preparation method of monoclinic tungsten trioxide
A technology of tungsten trioxide and monoclinic crystal, applied in the direction of tungsten oxide/tungsten hydroxide, nanotechnology, etc., to achieve good reproducibility, uniform particle size distribution, and avoid production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
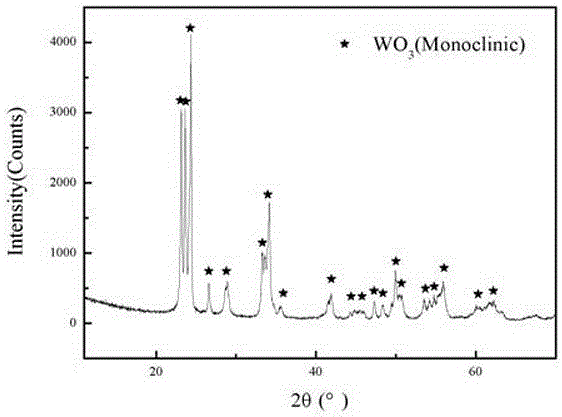
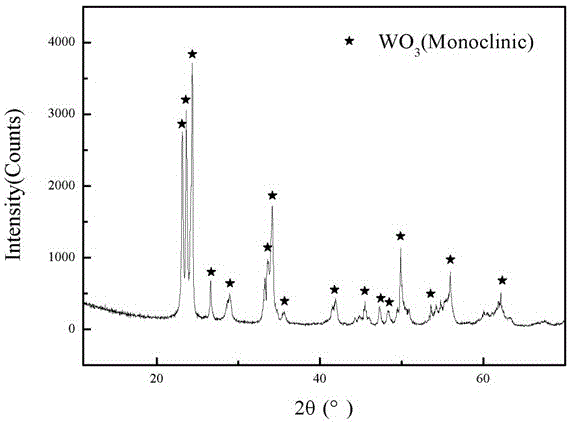
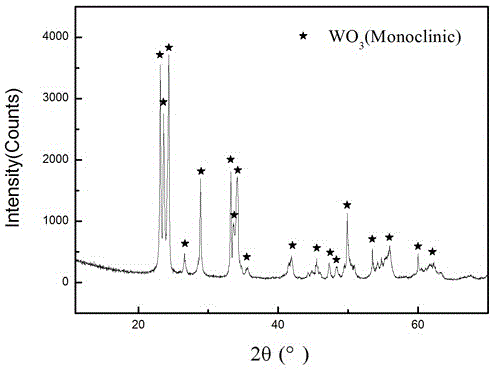
Image
Examples
preparation example Construction
[0024] A preparation method of monoclinic tungsten trioxide, which will be able to ionize WO in solution 4 2- The soluble tungstate (ammonium metatungstate is used as an example in this description) is prepared by hydrothermal method after stirring and mixing with nitric acid solution. Include the following steps:
[0025] (1) Measure a certain volume of commercially available nitric acid (63vol.% concentration, 1.4g / ml density) to prepare a nitric acid solution with a concentration of 12.14~14mol / L, and place it in a clean beaker.
[0026] (2) Weigh 303g of ammonium metatungstate powder [(NH 4 ) 6 h 2 W 12 o 40 ], where the ammonium metatungstate used is a white crystalline powder. Add to the beaker in step 1, stir for 10-30min to make it evenly mixed. During this process, the main reactions that take place are:
[0027] (NH 4 ) 6 h 2 W 12 o 40 +8H 2 O=6NH 4 + +12WO 4 2- +18H +
[0028] WO 4 2- +2H + =H 2 WO 4
[0029] (3) Immediately transfer the r...
Embodiment 1
[0038] (1) Weigh 303g of ammonium metatungstate powder [(NH 4 ) 6 h 2 W 12 o 40 ], where ammonium metatungstate is a white crystalline powder.
[0039] (2) Measure 347ml of commercially available nitric acid solution (mass concentration is 63%, density is 1.4g / ml.), put it in a clean beaker, and dilute the solution in this beaker to 400ml with deionized water.
[0040] (3) Add the ammonium metatungstate in step (1) into the above-mentioned beaker containing the nitric acid solution, and stir for 30 minutes to make it evenly mixed.
[0041] (4) Transfer the reaction system obtained in step (3) to a polytetrafluoroethylene-lined hydrothermal reaction kettle, place it in a vacuum drying oven, and react at 150°C for 20 hours, then take it out, and cool it at room temperature. A hydrothermal product is obtained.
[0042] (5) The hydrothermal reaction product obtained in step (4) is subjected to vacuum filtration, and the suction filtration is multiple suction filtration. Slo...
Embodiment 2
[0045] (1) Weigh 303g of ammonium metatungstate powder [(NH 4 ) 6 h 2 W 12 o 40 ], where ammonium metatungstate is a white crystalline powder.
[0046] (2) Measure 380ml of commercially available nitric acid solution (mass concentration: 63%, density: 1.4g / ml.), and place it in a clean beaker.
[0047] (3) Add the ammonium metatungstate in step (1) into the above-mentioned beaker containing the nitric acid solution, and stir for 30 minutes to make it evenly mixed.
[0048] (4) Transfer the reaction system obtained in step (3) to a polytetrafluoroethylene-lined hydrothermal reaction kettle, place it in a vacuum drying oven, and react at 170°C for 15 hours, then take it out, and cool it at room temperature. A hydrothermal product is obtained.
[0049] (5) The hydrothermal reaction product obtained in step (4) is subjected to vacuum filtration, and the suction filtration is multiple suction filtration. Slowly add distilled water into the funnel during the first suction fil...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com