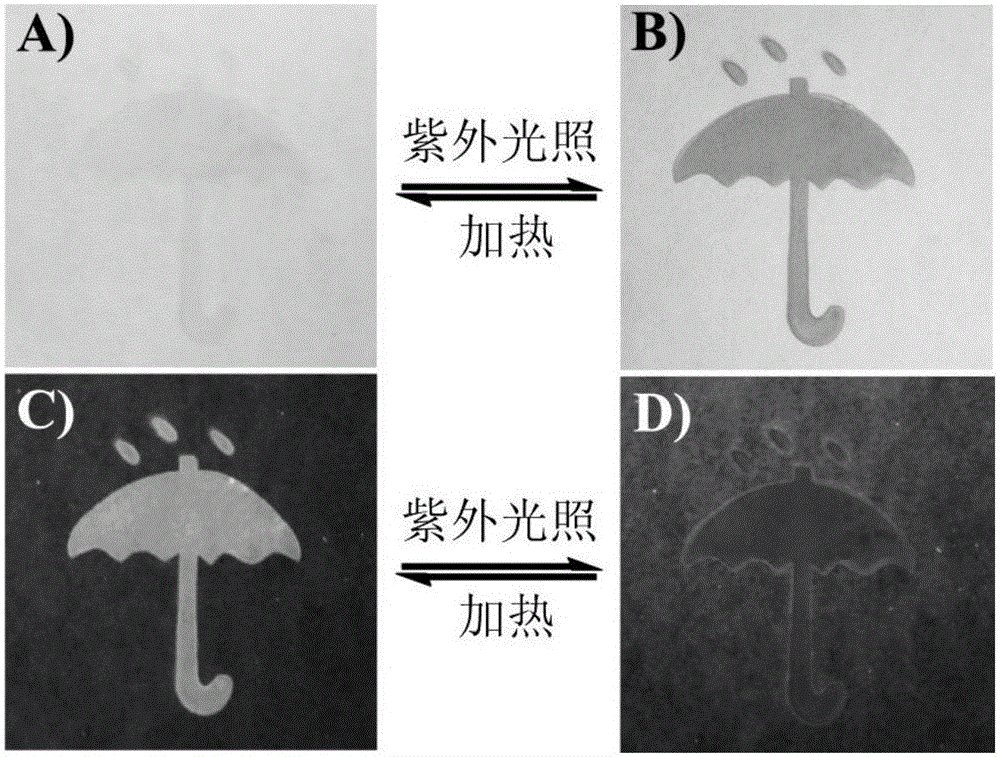
Reversible solid photochromic fluorescence ink material and application thereof
A photochromic and fluorescent ink technology, applied in the direction of color-changing fluorescent materials, inks, applications, etc., can solve the problems of limited practical application, poor resistance, instability, etc., and achieve the effect of excellent stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] When Ar is 9,10-distyryl anthracene (DSA) (n=2 in the structural formula), its molecular structure is as follows:
[0017]
[0018] Specific synthesis steps: Dissolve 1-carboxyethylindoline spiropyran molecule (2mmol, 0.76g) in 5mL of anhydrous dichloromethane, add 4-dimethylaminopyridine (3mg) and 9,10- Bis[4-hydroxystyryl]anthracene (1 mmol, 0.414 g). Cool down to 0°C, then slowly add dicyclohexylcarbodiimide (3mmol, 0.618g) dropwise into the reaction system, keep stirring at 0°C for 5min, then return to room temperature and stir for 10h.
[0019] Post-processing: first remove the insoluble precipitate in the reaction mixture by suction filtration, wash the filtrate twice with 0.5M hydrochloric acid and saturated sodium bicarbonate solution, separate the organic phase of dichloromethane, then dry it with anhydrous magnesium sulfate, and remove it by filtration. Anhydrous magnesium sulfate, the filtrate was evaporated to remove the solvent under reduced pressure, a...
Embodiment 2
[0022]
[0023] 1-Carboxyethylindoline spiropyran molecule (1mmol, 0.38g) was dissolved in 5mL of anhydrous dichloromethane, and 4-dimethylaminopyridine (1.5mg) and 9,10-[4- Hydroxystyryl]anthracene (1 mmol, 0.398 g). Cool down to 0°C, then slowly add dicyclohexylcarbodiimide (1.5mmol, 0.309g) dropwise into the reaction system, then keep stirring at 0°C for 5min, then return to room temperature and stir for 10h. The post-treatment was the same as in Example 1, and the finally obtained product DSA-SP was a yellow powder (0.46 g, 60%).
[0024]LC-MS(ESI): m / z: Calculated: 760.29, Found: 761.34 [M+H] + . Elemental analysis (calculated): C80.4% (80.51%), H5.4% (5.30%), N3.7% (3.68%).
Embodiment 3
[0026]
[0027] 1-Carboxyethylindoline spiropyran molecule (4mmol, 1.52g) was dissolved in 5mL of anhydrous dichloromethane, and 4-dimethylaminopyridine (6mg) and 4,4',4", 4"'-Tetrahydroxytetraphenylethylene (1 mmol, 0.396 g). Cool down to 0°C, then slowly add dicyclohexylcarbodiimide (6mmol, 1.236g) dropwise into the reaction system, then keep stirring at 0°C for 5min and return to room temperature and stir for 10h. The aftertreatment was the same as in Example 1, and the final product TPE-4SP was yellow powder (0.51 g, 28%).
[0028] LC-MS (ESI): m / z: Calculated: 1844.64, Found: 462.18 [M+4H] 4+ / 4. Elemental analysis (calculated): C71.4% (71.57%), H5.0% (5.02%), N6.0% (6.07%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com