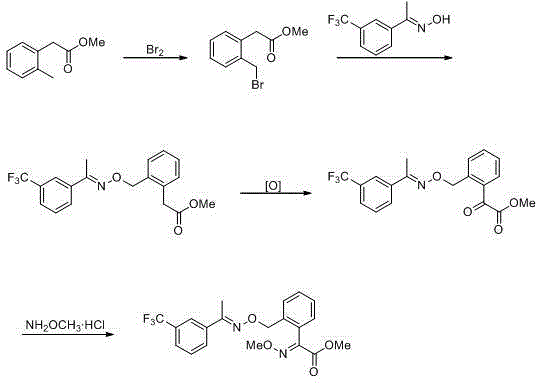
Trifloxystrobin synthesizing method
A technology for trifloxystrobin and compounds, which is applied in the field of organic synthesis reactions, can solve the problems of reducing the profit of industrialized production, being unsuitable for industrialization, and being dangerous, and achieves the effects of enhancing market competitiveness, protecting safety, and reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment one, ( E )-3-keto-4-(isonitroso)isochroman
[0037] A 50mL Schlenk bottle was subjected to anhydrous and oxygen-free treatment, and then added 1.48g (10mmol) o-hydroxymethylphenylacetic acid lactone, 1.236g (12mmol) TBN and 25mL anhydrous methanol, stirred in an ice bath for 10min, and then added dropwise Sodium methoxide (0.756g, 14mmol) in methanol (5mL) was stirred at room temperature for 24h. After the reaction, spin off the methanol, add 20 mL each of water and ethyl acetate, then use dilute hydrochloric acid to neutralize the system to a pH of about 7, extract three to four times with ethyl acetate, combine the organic phases, dry, spin dry, and then Purified by silica gel column chromatography to obtain 1.15 g of product with a yield of 65%. (TBN tert-butyl nitroso ester) 1 HNMR (600MHz, DMSO- d 6 ): δ =5.44(s,2H),7.43-7.51(m,3H),8.32(d, J =7.6Hz,1H),13.2(s,1H)ppm.
Embodiment 2
[0038] Embodiment two, ( E Preparation of )-3-keto-4-(methoxyimino)isochroman
[0039] To a clean 50mL round bottom flask, add 708mg (4mmol) ( E )-3-keto-4-(isonitroso)isochroman, 12mL acetone and 828mg (6mmol) potassium carbonate, a large amount of light yellow solid appeared after a while, stirred at room temperature for 0.5h, added dropwise 756mg (6mmol) sulfuric acid The acetone (3mL) solution of dimethyl ester was stirred at room temperature for 8h to stop the reaction, the acetone was rotated off, water and ethyl acetate were added, and ethyl acetate was extracted three to four times. The organic phases were combined, dried, spin-dried, and passed through a silica gel column Analysis and purification, to obtain 412mg product, productive rate 54%.
[0040] 1 HNMR (600MHz, DMSO- d 6 ): δ =4.13(s,3H),5.47(s,2H),7.45-7.47(m,2H),7.52-7.55(m,1H),8.18(d, J =7.8Hz, 1H)ppm.
Embodiment 3
[0041] Embodiment three, ( E ) Preparation of -2-chloromethyl-α-methoxyiminophenylacetic acid methyl ester
[0042] Add 320mg (1.68mmol) ( E )-3-keto-4-(methoxyimino)isochroman, 4mL of methanol, 2.998g (25.2mmol) of thionyl chloride was added dropwise in ice bath, then stirred at room temperature for 28h, quenched with water, ethyl acetate Extracted three to four times, combined the organic phases, dried, spin-dried, and then purified by silica gel column chromatography to obtain 296 mg of the product with a yield of 73%.
[0043] 1 HNMR (600MHz, CDCl 3 ): δ =3.87(s,3H),4.05(s,3H),4.43(s,2H),7.17(d, J =7.4Hz,1H),7.38-7.44(m,2H),7.51(d, J =7.5Hz, 1H)ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com